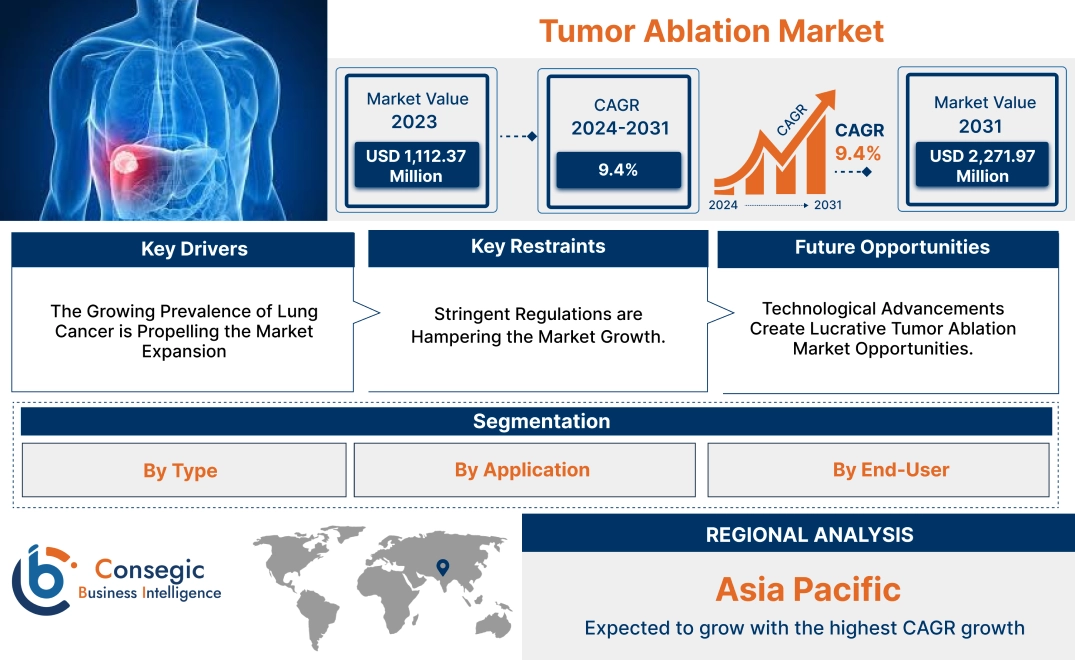
Tumor Ablation Market Size:
Tumor Ablation Market size is growing with a CAGR of 9.4% during the forecast period (2024-2031), and the market is projected to be valued at USD 2,271.97 Million by 2031 from USD 1,112.37 Million in 2023.
Tumor Ablation Market Scope & Overview:
Tumor ablation is a minimally invasive medical procedure that involves destroying cancerous cells within a tumor. This technique is used to treat tumors in various organs, including the liver, kidney, lung, pancreas, bone, and others. The procedure is performed using various methods, such as radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation among others. In RFA, high-frequency electrical currents heat and destroy tumor cells. MWA uses microwaves to generate heat, while cryoablation employs extreme cold to freeze and kill tumor cells. These techniques offer several advantages over traditional surgery, including smaller incisions, reduced recovery time, and less pain. As per the tumor ablation market analysis, it is particularly beneficial for patients who are not suitable for surgery or radiation therapy due to their overall health or the location and size of the tumor. The end-users are hospitals and clinics, cancer care centers, and ambulatory surgical centers.
How is AI Transforming the Tumor Ablation Market?
The integration of AI is considerably transforming the tumor ablation market. AI-powered systems are being used for improving procedure accuracy, personalization, and efficiency through real-time imaging, enhanced planning, and AI-driven robotic systems. Moreover, AI-powered systems are used to integrate imaging, clinical, and genomic data to help physicians precisely target tumors, minimize damage to surrounding healthy tissues, and personalize treatment plans for improved patient outcomes. This leads to more reliable and effective minimally invasive cancer treatments. Consequently, the above factors are anticipated to drive the market growth in upcoming years.
Tumor Ablation Market Dynamics - (DRO) :
Key Drivers:
The Growing Prevalence of Lung Cancer is Propelling the Market Expansion
The rising prevalence of various cancers, particularly lung cancer, is a significant driver for the tumor ablation market growth. Lung cancer remains one of the leading causes of cancer-related deaths worldwide, and traditional treatment options like surgery, chemotherapy, and radiation therapy are suitable for all patients. They offer a minimally invasive alternative for treating localized tumors, especially in cases where surgery is not feasible or when the patient's overall health condition limits their ability to tolerate more invasive procedures. As the global burden of cancer continues to increase, the demand for effective and less invasive treatment options is rising.
- For instance, according to the data published by the World Cancer Research Fund, in 2022, lung cancer is one of the leading causes of cancer incidence globally, with 2,480,675 cases.
Thus, the rise in the prevalence of lung cancer is boosting the use of ablation solutions to destroy the tumor, thus driving the tumor ablation market demand.
Development of Novel Bone Tumor Ablation Solutions is Driving the Market
The development of novel bone tumor ablation solutions is a significant driver for the growth of the tumor ablation market. Bone tumors are the abnormal growth of the tissues present in a bone. Traditional treatment options for bone tumors, such as surgery and radiation therapy, are invasive and have limitations. Novel minimally invasive approaches to treat bone tumors are being introduced, especially in cases where surgery is not feasible or when the tumor is located in a difficult-to-reach area.
These techniques such as radiofrequency ablation (RFA), microwave ablation (MWA), and others effectively destroy tumor tissues while minimizing damage to surrounding healthy tissues. As research and development continue to advance, new and improved ablation technologies are emerging, further expanding the treatment options for bone tumors. Various manufacturers are introducing novel ablation solutions to destroy bone tumors.
- For instance, in September 2022, Stryker's OptaBlate bone tumor ablation system received FDA clearance, for bone cancer treatment. This innovative technology offers a less invasive approach to treating bone tumors, particularly in cases where traditional surgery is feasible or desirable.
Thus, the development of novel ablation solutions for tumors is driving tumor ablation market growth.
Key Restraints :
Stringent Regulations are Hampering the Market Growth.
Stringent regulatory requirements significantly hinder the tumor ablation market demand. The development and commercialization of new ablation technologies require rigorous clinical trials and regulatory approvals to ensure their safety and efficacy. The complex regulatory landscape, varying across different countries significantly delays the market entry of innovative ablation devices.
Moreover, the stringent quality control standards and manufacturing regulations imposed by regulatory agencies increase the costs associated with product development and commercialization. Furthermore, post-market surveillance and safety monitoring requirements add to the regulatory burden. Companies must continuously monitor the safety and efficacy of their products, which becomes costly and resource-intensive, especially for smaller players. Thus, these factors contribute to the slower adoption of new ablation technologies and hinder the overall growth of the market.
Future Opportunities :
Technological Advancements Create Lucrative Tumor Ablation Market Opportunities.
Technological advancements are significantly creating tumor ablation market opportunities for the treatment of tumors. The development of innovative ablation techniques, such as advanced radiofrequency ablation (RFA) with multiple probes, has expanded the treatment options for various types of tumors. These techniques offer minimally invasive approaches with shorter recovery times and fewer side effects compared to traditional surgical procedures. Manufacturers are introducing advanced solutions for the treatment of various tumors.
- For instance, in March 2024, Medtronic received FDA clearance for the OsteoCool™ 2.0 bone tumor ablation system that represents a significant advancement in the treatment of painful bone tumors. This innovative system offers faster ablation times, increased power output, and the ability to use up to four probes simultaneously. These enhancements enable more efficient and effective treatment of bone tumors, reducing procedure time and improving patient outcomes.
Overall, as technology continues to evolve, further refinement in ablation techniques leads to improved patient outcomes and increased market demand for ablation devices and procedures.
Tumor Ablation Market Segmental Analysis :
By Type:
Based on type, the market is categorized into radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation, percutaneous ethanol ablation (PEI), and others.
Trends in the Type:
- Real-time imaging techniques like ultrasound, CT, and MRI allow for precise targeting of tumors, minimizing damage to surrounding healthy tissue.
- Emerging technologies such as microwave ablation, laser ablation, and high-intensity focused ultrasound (HIFU) are offering more precise and effective treatments.
The radiofrequency ablation (RFA) segment accounted for the largest tumor ablation market share in 2023.
- Radiofrequency ablation (RFA) is a minimally invasive procedure used to treat tumors in various organs, including the liver, kidney, lung, and bone among others.
- During RFA, a thin probe is inserted into the tumor, and high-frequency electrical currents are delivered through the probe to heat and destroy the tumor cells. This heat generation causes cell death and coagulation of tissue proteins, effectively eliminating the tumor.
- RFA offers several advantages over traditional surgery, such as shorter hospital stays, less pain, and a quick recovery time.
- It is particularly useful for patients who are not suitable for surgery or radiation therapy due to their overall health or the location and size of the tumor.
- Manufacturers are introducing novel RFA solutions, for destroying tumors.
- For instance, in April 2023, Compal Electronics introduced the AblatePal. This innovative system utilizes radiofrequency ablation (RFA) technology to effectively destroy tumor cells. The AblatePal system is designed to offer precise and efficient tumor ablation, providing a less invasive alternative to traditional surgical procedures.
- Thus, as per the analysis, the use of RFA for ablation of cancer treatment is driving the tumor ablation market trends.
The cryoablation segment is expected to grow at the fastest CAGR over the forecast period.
- Cryoablation is a minimally invasive procedure that uses extreme cold to freeze and destroy abnormal cells or diseased tissue, including cancerous tumors.
- During the procedure, a thin, needle-like probe, called a cryoprobe, is inserted into the tumor.
- A freezing agent, such as liquid nitrogen or argon gas, is then delivered through the probe, causing the tissue to freeze and form ice crystals. This freezing process damages the cells, leading to their death.
- The freezing and thawing cycles are repeated several times to ensure the breakdown of the tumor.
- Cryoablation is often used to treat tumors in the liver, kidney, lung, bone, and prostate.
- Thus, as per the analysis, the use of cryoablation is driving the development of the segment in the coming years.
By Application:
Based on applications, the market is categorized into liver cancer, lung cancer, kidney cancer, bone cancer, and others
The liver cancer segment accounted for the largest tumor ablation market share in 2023.
- Tumor ablation is a minimally invasive procedure used to treat liver cancer. It involves destroying cancerous cells using various techniques, including radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation among others.
- In RFA, high-frequency electrical currents are delivered through a probe inserted into the tumor, generating heat that destroys the cancer cells present in the liver.
- MWA uses microwave energy to heat and destroy tumor tissue in the liver.
- Cryoablation involves freezing the liver tumor cells with extreme cold, causing them to die.
- These techniques offer several advantages over traditional surgery, including smaller incisions, shorter recovery time, and less pain. Tumor ablation is particularly beneficial for patients who are not suitable for surgery or radiation therapy due to their overall health or the location and size of the liver tumor.
- Various manufacturers are introducing novel solutions for liver tumors.
- For instance, Johnson & Johnson provides the NEUWAVE™ Microwave System which is intended for percutaneous, open, and laparoscopic ablation of soft tissue, specifically for the partial or complete ablation of non-resectable liver tumors.
- Thus, based on the analysis, the rising trends for the use of ablation solutions for liver cancer are influencing the tumor ablation market expansion.
The breast cancer segment is expected to grow at the fastest CAGR over the forecast period.
- Breast cancer is a disease that occurs when cells in the breast grow uncontrollably, forming a tumor. It is one of the most common types of cancer affecting women worldwide.
- While traditional surgical options like lumpectomy and mastectomy remain the primary treatments, ablation therapies like radiofrequency ablation (RFA) and cryoablation are being explored for specific cases.
- RFA and cryoablation involve using heat or extreme cold to destroy tumor cells.These minimally invasive techniques are considered for small, early-stage breast tumors in select patients.
- Overall, as research continues to evolve, further advancements in ablation technologies are being introduced, expanding their role in breast cancer treatment, thus driving the tumor ablation market trends.
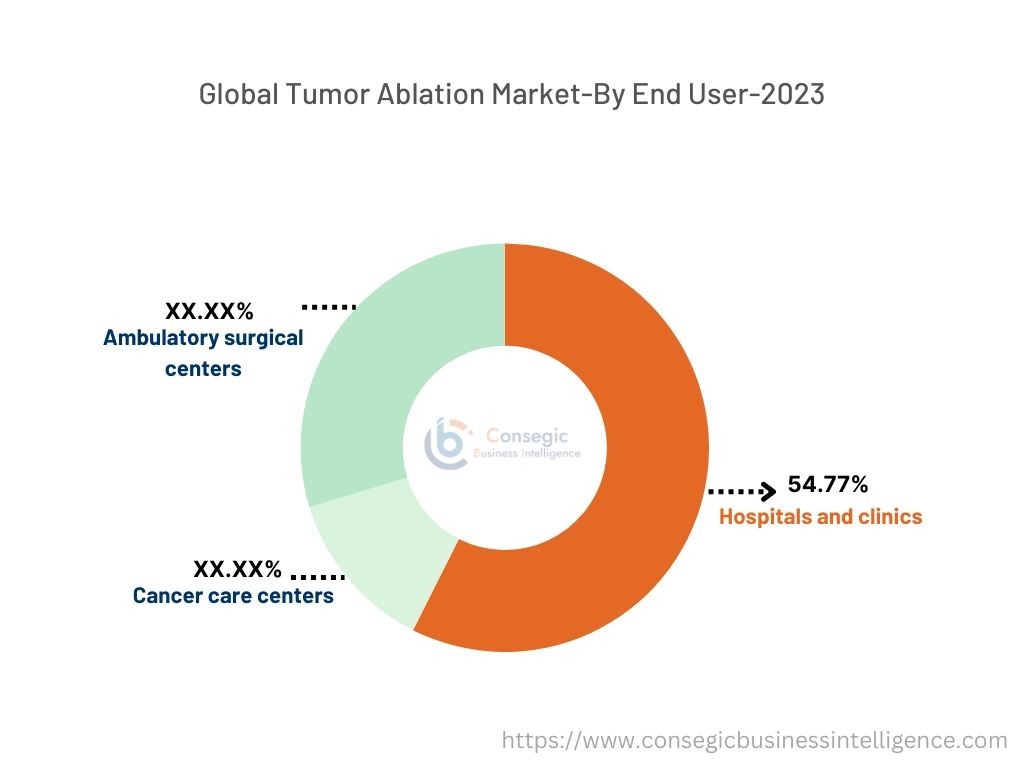
By End User:
Based on end users, the market is categorized into hospitals and clinics, cancer care centers, and ambulatory surgical centers.
Trends in the End User:
- There is a growing trend for minimally invasive procedures in places equipped with advanced solutions.
- The growing trends for same-day procedures are influencing the segment
The hospitals and clinics segment accounted for the largest market share of 54.77% in the year 2023.
- Hospitals and clinics serve a crucial role as primary centers for the comprehensive treatment of cancer, offering a sophisticated ecosystem of medical expertise and advanced technologies.
- Modern hospital-based management monitors for early signs of this condition. They play a major and crucial role in helping patients by providing convenient, affordable, and easy access to the treatment of cancer.
- The inpatient treatment approach in hospitals has evolved to encompass a multidisciplinary approach making availability of cancer specialists in a single place. Moreover, hospitals increasingly invest in facilities equipped with specialized equipment and offer advanced solutions.
- For instance, in 2022, American Hospital, Dubai made a significant stride in minimally invasive cancer treatment by performing the first cryoablation procedure for kidney cancer in the Middle East and North Africa (MENA) region. This innovative technique offers a less invasive alternative to traditional surgical methods, providing patients with a quick recovery time and reduced risk of complications.
- Thus, as per the analysis, owing to the aforementioned factors, hospitals serve a pivotal role in the treatment, providing comprehensive care in the
The ambulatory surgical center segment is expected to grow at the fastest CAGR over the forecast period.
- Ambulatory surgery centers, or ASCs, are modern healthcare facilities focused on providing same-day care and ablation procedures. These settings offer a convenient and cost-effective alternative to traditional hospitals for a variety of cancer procedures.
- ASCs are designed to handle procedures that do not require overnight hospitalization, allowing patients to go home on the same day. Ambulatory surgical centers are increasingly playing a role in managing patients.
- ASCs are equipped with novel technology and experienced medical professionals to ensure the safety and efficacy of ablation procedures.
- These minimally invasive procedures, such as radiofrequency ablation (RFA) and cryoablation, target and destroy tumor cells using heat or cold, respectively. The advantages of performing tumor ablation in ASCs include shorter recovery times, reduced hospital stays, and lower costs compared to traditional hospital settings.
- Overall, as per the tumor ablation market analysis, as technology advances and patient preferences evolve, ASCs are becoming a preferred venue for delivering high-quality, minimally invasive cancer care.
Regional Analysis:
The regional segment includes North America, Europe, Asia Pacific, the Middle East and Africa, and Latin America.
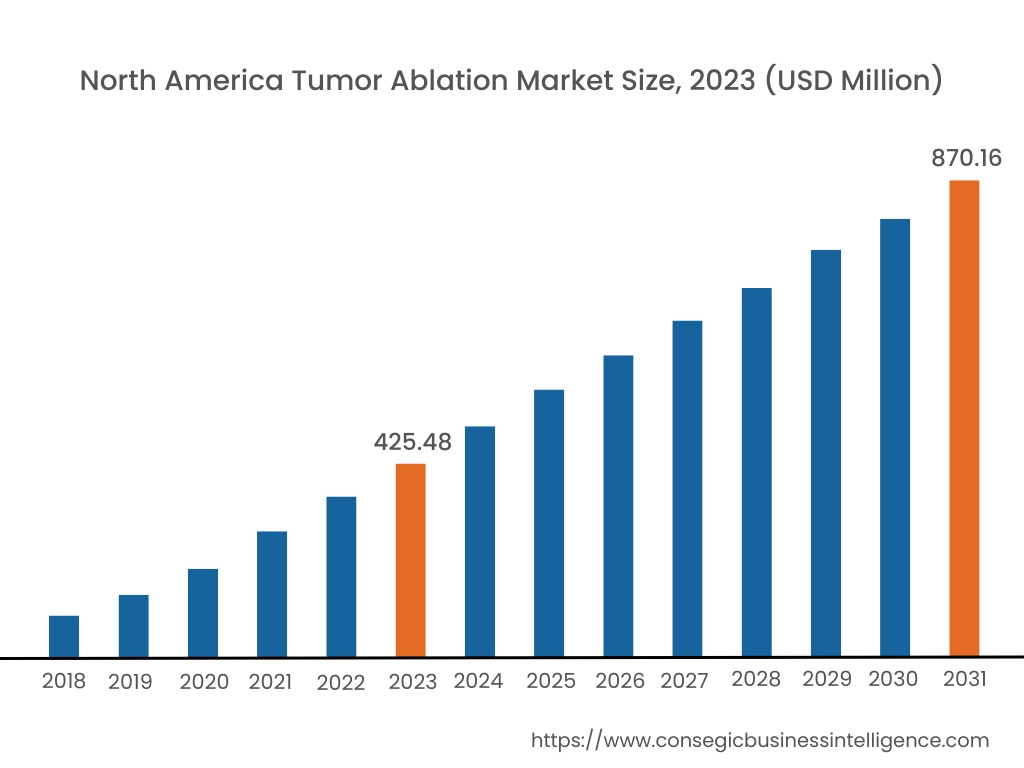
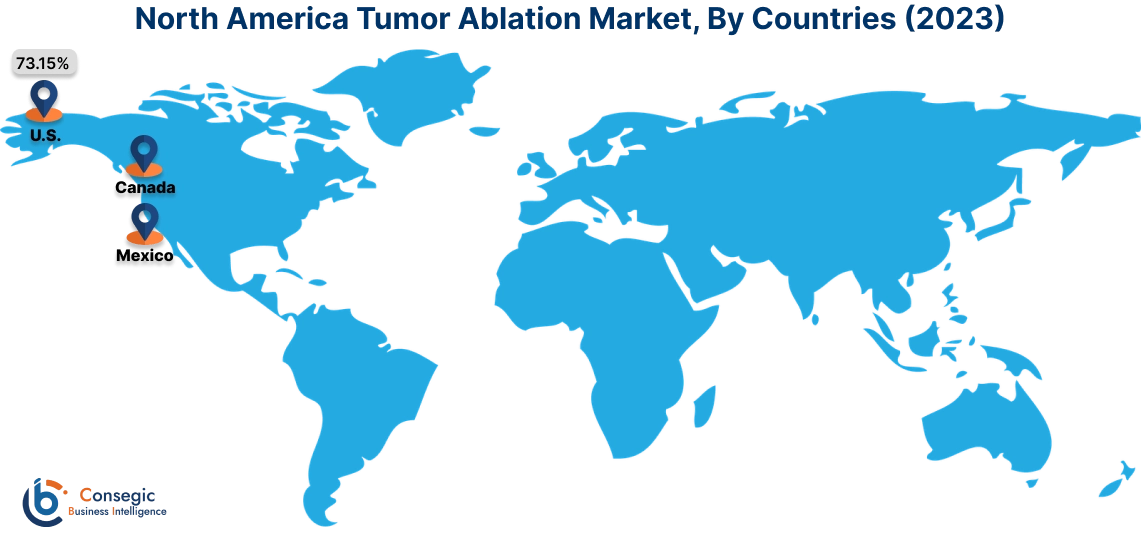
In 2023, North America accounted for the highest market share at 38.25% and was valued at USD 425.48 Million and is expected to reach USD 870.16 Million in 2031. In North America, the U.S. accounted for the highest market share of 73.15% during the base year of 2023. Tumor ablation in North America is a well-established and widely practiced minimally invasive procedure. The region has a strong focus on technological advancements and the early adoption of innovative medical techniques. This has led to significant advancements in ablation technologies, such as radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation, among others. These techniques are increasingly being used to treat a variety of tumors, including those in the liver, kidney, lung, and bone among others. The strong healthcare infrastructure, experienced medical professionals, and supportive regulatory environment in North America have contributed to the widespread adoption and successful implementation of tumor ablation procedures. Various manufacturers are introducing their subsidiaries that provide ablation solutions for tumors in North American countries.
- For instance, in 2023, South Korean medical device leader, STARmed Co., Ltd., solidified its international presence with the launch of its U.S. subsidiary, STARmed America which offers ablation solutions for various applications.
Thus, the advancements in ablation procedures for cancer treatment in the U.S. are influencing tumor ablation market expansion.
The Asia Pacific market for Tumor Ablations is experiencing the fastest growth with a CAGR of 10.4% over the forecast period. Tumor ablation in the Asia Pacific region is a rapidly growing field, driven by increasing cancer incidence rates and advancements in medical technology. Countries like China, India, Japan, and South Korea are at the forefront of adopting minimally invasive techniques, including ablation for tumors. This region offers a significant market opportunity for medical device manufacturers and healthcare providers. The region is witnessing significant advancements in medical technology, including the development of advanced ablation systems and imaging techniques. Additionally, governments in many Asian countries are investing in healthcare infrastructure and promoting the adoption of minimally invasive procedures. Overall, rising disposable incomes and government initiatives are driving increased healthcare expenditure, making advanced treatments more accessible in the Asia Pacific region.
Tumor ablation in Europe is a well-established and widely practiced minimally invasive procedure. European countries have a strong focus on healthcare innovation and early adoption of new medical technologies. The European Union's regulatory framework, particularly the Medical Device Regulation (MDR), plays a crucial role in ensuring the safety and efficacy of ablation devices. Strict regulatory standards have led to the development of high-quality and innovative ablation systems. Additionally, the strong collaboration between healthcare providers, researchers, and industry partners in Europe has facilitated the development and implementation of advanced ablation techniques. As a result, Europe has become a significant player in the global tumor ablation market, contributing to the development and dissemination of innovative treatment options for patients with cancer.
Middle East and Africa have made significant strides in healthcare infrastructure and technological advancements, and the adoption of tumor ablation procedures is still evolving in these regions. The rising prevalence of cancer in these regions is driving the requirement for effective and minimally invasive treatment options. They offer a promising approach for treating various types of cancer, especially in cases where traditional surgery is not feasible. Governments in many Middle Eastern and African countries are investing heavily in healthcare infrastructure and medical technology. This increased investment is leading to the establishment of specialized cancer centers and the availability of advanced ablation techniques. As healthcare awareness increases, patients are becoming more informed about minimally invasive treatment options. This growing awareness is driving the demand for tumor ablation procedures.
The adoption of tumor ablation in Latin America is still emerging compared to regions such as North America and Europe. The increasing prevalence of cancer and the growing awareness of minimally invasive treatment options are driving the demand for ablation procedures for tumors. As healthcare spending increases, more resources are being allocated to advanced medical technologies, including tumor ablation. Growing awareness among healthcare providers and patients about the benefits of minimally invasive procedures is driving demand. The development of advanced ablation technologies, such as radiofrequency ablation and microwave ablation, is making these procedures more accessible and effective. Thus, as the Latin American market for tumor ablation is developing, it holds significant potential for growth in the coming years.
Top Key Players & Market Share Insights:
The tumor ablation market is highly competitive with major players providing precise treatment to the national and international markets. Key players are adopting several strategies in research and development (R&D) and product innovation to hold a strong position in the global tumor ablation market. Key players in the tumor ablation industry include-
- Medtronic (United States)
- AngioDynamic (United States)
- BVM Medical (United Kingdom)
- Merit Medical Systems (United States)
- Stryker (United States)
- Olympus (Japan)
- Johnson & Johnson MedTech (United States)
- Varian (United States)
- IceCure Medical (Israel)
- Boston Scientific Corporation (United States)
Recent Industry Developments:
Partnerships:
- In 2023, CLS Americas and Focalyx partnered to deliver an integrated solution for image-guided focal laser ablation of prostate tumors, combining CLS's TRANBERG Thermal Therapy System with Focalyx's Fusion image guidance system.
Product Approvals:
- In September 2022, Stryker's OptaBlate bone tumor ablation system received FDA clearance, for bone cancer treatment. This innovative technology offers a less invasive approach to treating bone tumors, particularly in cases where traditional surgery is feasible or desirable.
Tumor Ablation Market Report Insights :
| Report Attributes | Report Details |
| Study Timeline | 2018-2031 |
| Market Size in 2031 | USD 2,271.97 Million |
| CAGR (2024-2031) | 9.4% |
| By Type |
|
| By Application |
|
| By End-User |
|
| By Region |
|
| Key Players |
|
| North America | U.S. Canada Mexico |
| Europe | U.K. Germany France Spain Italy Russia Benelux Rest of Europe |
| APAC | China South Korea Japan India Australia ASEAN Rest of Asia-Pacific |
| Middle East and Africa | GCC Turkey South Africa Rest of MEA |
| LATAM | Brazil Argentina Chile Rest of LATAM |
| Report Coverage |
|
Key Questions Answered in the Report
How big is the tumor ablation market? +
In 2023, the tumor ablation market is USD 1,112.37 Million.
Which is the fastest-growing region in the tumor ablation market? +
Asia Pacific is the fastest-growing region in the tumor ablation market.
What specific segmentation details are covered in the tumor ablation market? +
Type, application, and end-user segmentation details are covered in the tumor ablation market.
Who are the major players in the tumor ablation market? +
Medtronic (United States), AngioDynamic (United States), BVM Medical (United Kingdom), Merit Medical Systems (United States), Stryker (United States), Olympus (Japan), Johnson & Johnson MedTech (United States), Varian (United States), IceCure Medical (Israel) and Boston Scientific Corporation (United States).