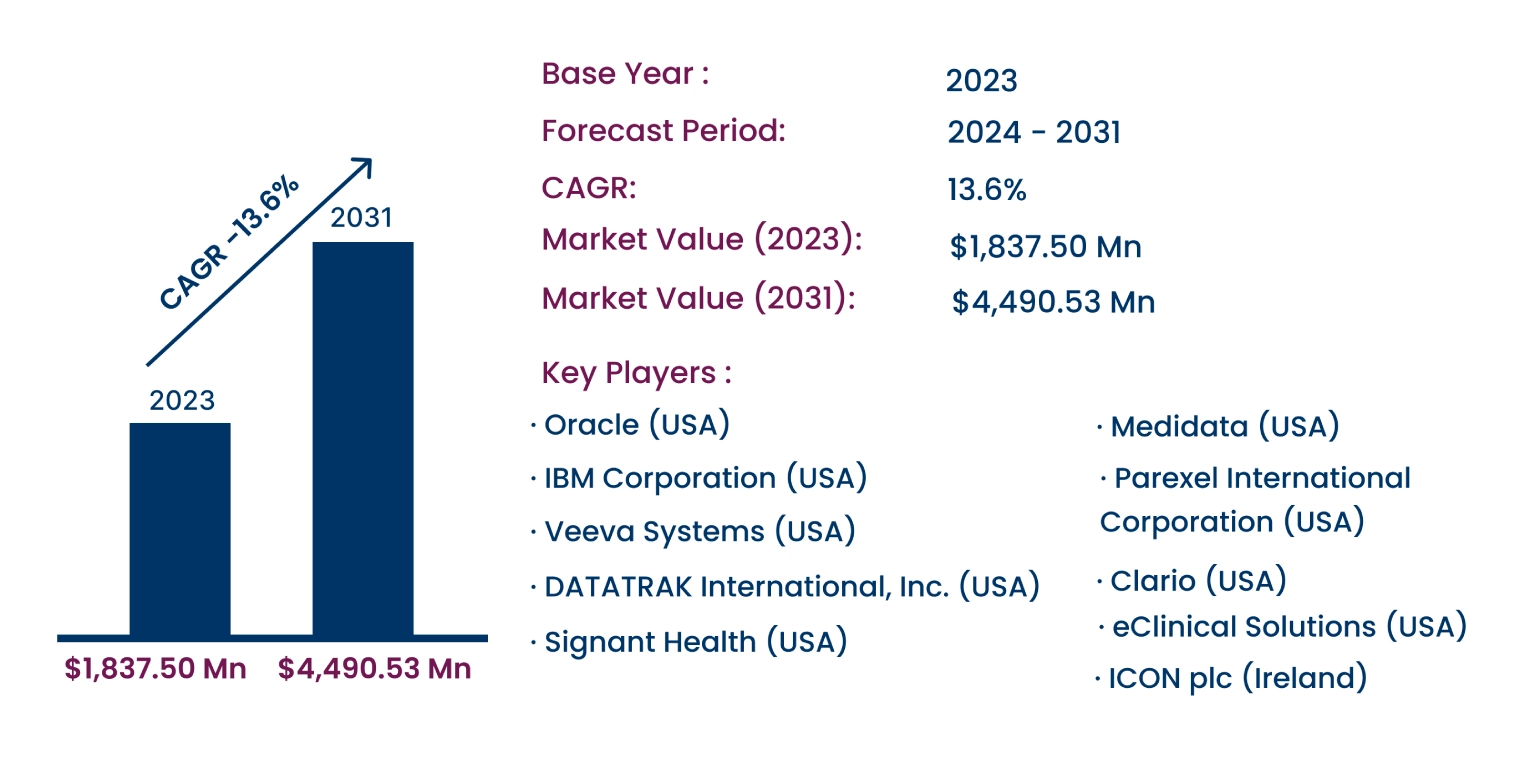
Global Clinical Data Management Systems Market to Reach USD 4,490.53 Million by 2031 | CAGR of 13.6%
Category : Healthcare | Published Date : Oct 2024 | Type : Press Release
Clinical Data Management Systems Market Scope & Overview:
Clinical Data Management (CDM) systems are software platforms used in the collection, management, and integration of clinical trial data. CDM systems ensure the accuracy and regulatory compliance of data collected during clinical trials, helping streamline data entry, validation, and storage processes. They also track and maintain detailed records of data edits and approvals through audit trails. CDM systems are widely adopted by pharmaceutical firms, contract research organizations (CROs), academic institutions, and medical facilities to ensure high-quality, error-free data while reducing the time required for data collection.
Consegic Business Intelligence analyzes that the Global Clinical Data Management Systems Market size was valued at USD 1,837.50 Million in 2023 and is projected to register a CAGR of 13.6% to reach USD 4,490.53 Million by 2031.
This report comprises the Clinical Data Management Systems Market Share, Size & Industry Analysis, By Type (Licensed Enterprise Software, Web-Based Software, Cloud-Based Software), By Component (Software, Services), By Mode of Delivery (On-Premise, Cloud-Based, Web-Based), By Application (Pharmacovigilance, Clinical Trial Analysis, Regulatory Compliance, Medical Research), By End-User (Pharmaceutical & Biotechnology Companies, Contract Research Organizations (CROs), Academic Research Institutes, Hospitals & Healthcare Providers), By Region (North America, Europe, Asia Pacific, Middle East & Africa, Latin America), and Forecast, 2024-2031.
This report contains detailed information on Clinical Data Management Systems Market Trends, Opportunities, Value, Growth Rate, Segmentation, Geographical Coverage, Company Profile, In-depth Expert Analysis, Revenue Forecast, Competitive Landscape, Growth Factors, Restraint or Challenges, Environment & Regulatory Landscape, PESTLE Analysis, PORTER Analysis, Key Technology Landscape, Value Chain Analysis, and Cost Analysis.
The growing volume of clinical trials has led to increased adoption of CDM systems. The shift toward electronic data capture (EDC) and the integration of artificial intelligence (AI) has enhanced the efficiency and accuracy of CDM systems, reducing trial timelines and increasing demand for data management solutions in clinical research. The increasing adoption of cloud-based CDM systems presents new growth opportunities, as cloud solutions offer scalability, cost-efficiency, and enhanced collaboration among clinical stakeholders. Cloud-based CDM systems also support decentralized trials, enabling data collection from diverse patient locations.
Segmental Analysis :
Based on the type, the market is bifurcated into Licensed Enterprise Software, Web-Based Software, and Cloud-Based Software.
- Cloud-based software accounted for the largest market share in 2023 due to its scalability and ability to handle large volumes of data efficiently.
- It is also expected to witness the fastest growth due to its cost-effectiveness and flexible deployment models.
Based on the component, the market is segmented into Software and Services.
- Software held the largest market share in 2023, driven by the growing demand for automation in clinical trials and the need for accurate data management.
- Services are projected to grow at the fastest rate, with the trend toward outsourcing data management tasks to specialized providers.
Based on the mode of delivery, the market is segmented into On-Premise, Cloud-Based, and Web-Based.
- Cloud-based delivery dominated the market in 2023, offering enhanced security, real-time data access, and compliance with regulatory standards.
- It is also expected to be the fastest-growing segment due to its ability to support decentralized trials and advanced analytics.
Based on the application, the market is segmented into Pharmacovigilance, Clinical Trial Data Management, Regulatory Compliance, and Medical Research.
- Clinical Trial Data Management was the largest segment in 2023, supported by the growing complexity of clinical trials, especially in oncology and rare diseases.
- Pharmacovigilance is projected to grow at the fastest rate, driven by the increasing demand for drug safety monitoring during clinical trials.
Based on regions, the global market is segmented into North America, Europe, Asia Pacific, Middle East & Africa, and Latin America.
- North America held the largest market share at 40.15% in 2023, valued at USD 737.76 Million, driven by advanced healthcare infrastructure and strict regulatory requirements in the U.S.
- The Asia Pacific region is expected to witness the fastest CAGR of 14.6% from 2024 to 2031, driven by the rapid growth of the pharmaceutical and biotechnology sectors in countries such as China, Japan, and India.
| Report Attributes | Report Details |
| Study Timeline | 2018-2031 |
| Market Size in 2031 | USD 4,490.53 Million |
| CAGR (2024-2031) | 13.6% |
| By Type | Licensed Enterprise Software, Web-Based Software, Cloud-Based Software |
| By Component | Software, Services |
| By Mode of Delivery | On-Premise, Cloud-Based, Web-Based |
| By Application | Pharmacovigilance, Clinical Trial Analysis, Regulatory Compliance, Medical Research |
| By End-User | Pharmaceutical & Biotechnology Companies, Contract Research Organizations (CROs), Academic Research Institutes, Hospitals & Healthcare Providers |
| By Region | North America(U.S., Canada, Mexico) Europe(U.K., Germany, France, Spain, Italy, Russia, Benelux, Rest of Europe) APAC(China, South Korea, Japan, India, Australia, ASEAN, Rest of Asia-Pacific) Middle East & Africa(GCC, Turkey, South Africa, Rest of MEA) LATAM(Brazil, Argentina, Chile, Rest of LATAM) |
Top Key Players & Competitive Landscape :
The competitive landscape encompasses major innovators, aftermarket service providers, industry giants, and niche players, all of which are thoroughly examined by Consegic Business Intelligence in terms of their strengths, weaknesses, and value-addition potential. This report includes detailed profiles of key players, market share analysis, mergers and acquisitions, resulting market fragmentation, and emerging partnership trends and dynamics.
List of prominent players in the Clinical Data Management Systems Industry:
- Oracle (USA)
- Medidata (USA)
- IBM Corporation (USA)
- Parexel International Corporation (USA)
- Veeva Systems (USA)
- Clario (USA)
- DATATRAK International, Inc. (USA)
- eClinical Solutions (USA)
- Signant Health (USA)
- ICON plc (Ireland)
Recent Industry Developments :
- In September 2024, the European Organization for Research and Treatment of Cancer (EORTC) extended its partnership with Medidata to facilitate oncology clinical trials, improving trial efficiency.
- In April 2024, GE Health completed the acquisition of MIM Software, enhancing its medical-tech portfolio with imaging analytics solutions.
- In February 2024, Revvity Signals launched Signals Clinical Solution, a SaaS platform designed to centralize clinical trial data management.
- In December 2023, Thermo Fisher Scientific launched CorEvidence, a cloud-based platform for optimizing pharmacovigilance case processing.
- In November 2023, Everest Clinical Research acquired August Research, expanding its global reach and presence in the European clinical trials market.