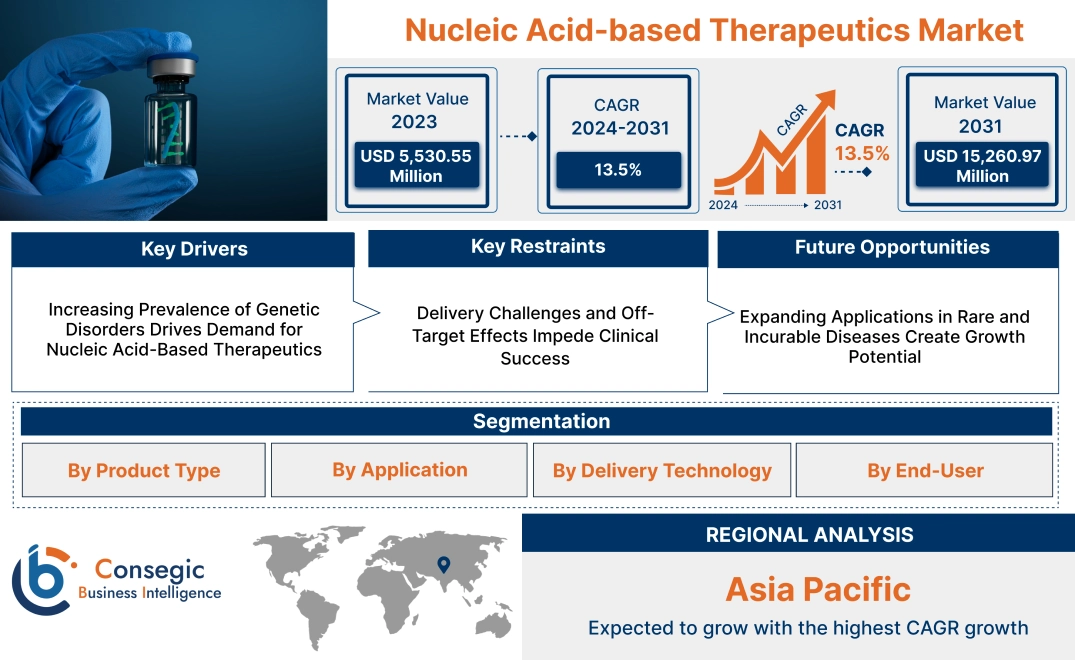
Nucleic Acid-based Therapeutics Market Size:
Nucleic Acid-based Therapeutics Market size is estimated to reach over USD 15,260.97 Million by 2031 from a value of USD 5,530.55 Million in 2023 and is projected to grow by USD 6,180.17 Million in 2024, growing at a CAGR of 13.5% from 2024 to 2031.
Nucleic Acid-based Therapeutics Market Scope & Overview:
The nucleic acid-based therapeutics are therapies that utilize nucleic acids such as DNA, RNA, and oligonucleotides to modulate gene expression and treat various diseases at the molecular level. These therapeutics include RNA interference (RNAi), antisense oligonucleotides, aptamers, and gene therapies designed to target the root cause of genetic and acquired diseases. Key characteristics include high specificity, the ability to silence disease-causing genes, and potential applications across a broad range of therapeutic areas. The benefits of these therapeutics include precise targeting of disease pathways, reduced off-target effects, and promising outcomes for conditions previously considered untreatable. Applications cover genetic disorders, cancer, infectious diseases, and cardiovascular conditions. End-users include pharmaceutical and biotechnology companies, research institutes, and healthcare providers, driven by advancements in genomic technologies, increasing prevalence of chronic diseases, and rising investments in personalized medicine.
Nucleic Acid-based Therapeutics MarketDynamics - (DRO) :
Key Drivers:
Increasing Prevalence of Genetic Disorders Drives Demand for Nucleic Acid-Based Therapeutics
The rising prevalence of genetic disorders, such as cystic fibrosis, Duchenne muscular dystrophy, Huntington’s disease, and sickle cell anemia, is a key driver for the market. These disorders are often caused by specific mutations or dysfunctions in genes, making nucleic acid-based therapies uniquely suited to address their underlying causes. Therapeutics like antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), and DNA-based therapeutics work by modulating gene expression, correcting mutations, or silencing harmful genes. For example, therapies like Spinraza (nusinersen) for spinal muscular atrophy have demonstrated the potential of nucleic acid-based drugs to transform the standard of care for genetic conditions. Advances in genomics and next-generation sequencing have also improved the identification of genetic targets, enabling the development of highly specific and personalized therapies. As awareness of genetic conditions grows and more effective diagnostic tools become available, the nucleic acid-based therapeutics market demand for targeted nucleic acid-based therapies is expected to rise significantly.
Key Restraints :
Delivery Challenges and Off-Target Effects Impede Clinical Success
One of the most significant challenges in the nucleic acid-based therapeutics market is the efficient and safe delivery of these therapies. Nucleic acids, including RNA and DNA, are inherently unstable in the physiological environment and are rapidly degraded by nucleases in the bloodstream. Ensuring that these molecules reach their target cells without being broken down or cleared by the immune system is a major obstacle. Delivery systems, such as lipid nanoparticles (LNPs) and viral vectors, have advanced significantly, but challenges remain in ensuring consistent bioavailability and targeted delivery.
Additionally, off-target effects, where the therapeutic molecules interact with unintended genes or proteins, can lead to severe safety concerns. Such interactions may result in unanticipated toxicities or immune responses, which can compromise the outcomes of clinical trials. Mitigating these effects requires extensive preclinical testing, sophisticated delivery mechanisms, and precise targeting strategies, which add complexity and cost to the drug development process. These challenges remain a significant hurdle to the broader adoption of nucleic acid-based therapeutics.
Future Opportunities :
Expanding Applications in Rare and Incurable Diseases Create Growth Potential
The increasing focus on rare and incurable diseases presents a transformative growth opportunity for the nucleic acid-based therapeutics market expansion. These therapies offer targeted and often life-changing solutions for conditions that lack effective treatments by addressing the disease at its genetic or molecular root. Rare neurological disorders, such as amyotrophic lateral sclerosis (ALS), spinal muscular atrophy (SMA), and Rett syndrome, are being actively explored for RNA-based therapies, including ASOs and siRNAs. For instance, Spinraza and Zolgensma have set benchmarks in treating SMA, showcasing the potential of nucleic acid-based solutions to address previously untreatable conditions.
The designation of orphan drugs, along with government incentives such as tax benefits and extended market exclusivity, is encouraging pharmaceutical companies to invest in this space. Moreover, advancements in precision medicine and bioinformatics are enabling faster identification of genetic mutations associated with rare diseases, accelerating the development of these therapies. The growing emphasis on personalized healthcare and unmet medical needs is expected to drive the adoption of new nucleic acid-based therapeutics market opportunities, and is unlocking new in both clinical research and commercial applications.
Nucleic Acid-based Therapeutics Market Segmental Analysis :
By Product Type:
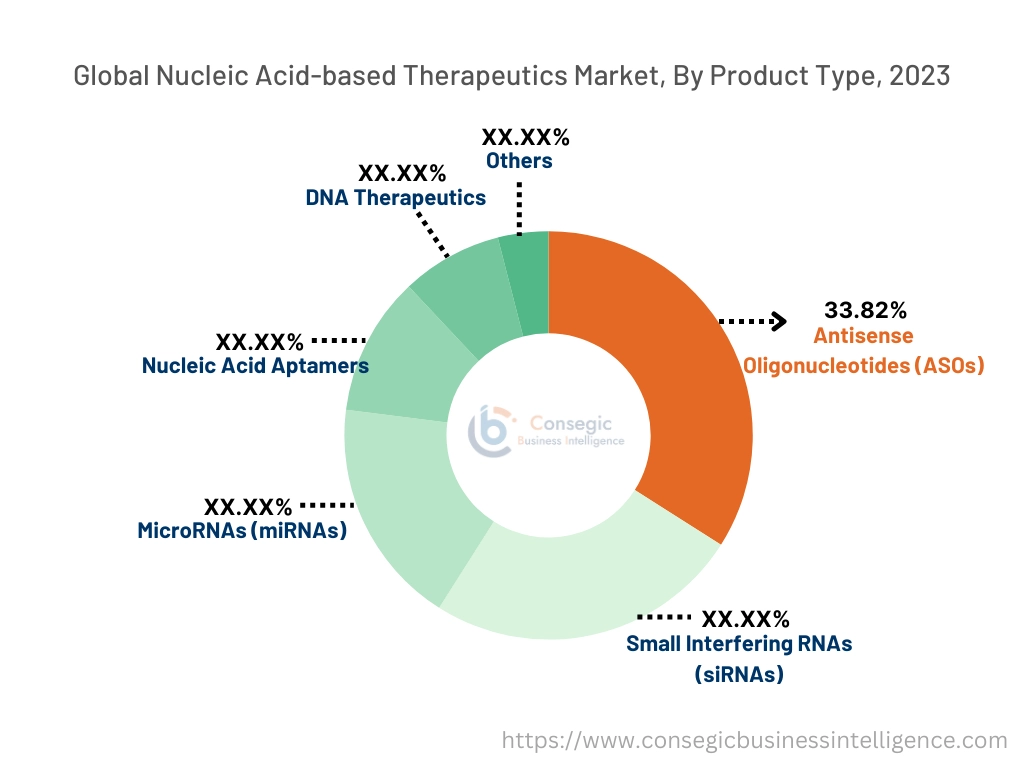
Based on product type, the market is segmented into Antisense Oligonucleotides (ASOs), Small Interfering RNAs (siRNAs), MicroRNAs (miRNAs), Nucleic Acid Aptamers, DNA Therapeutics, and Others.
The antisense oligonucleotides (ASOs) segment accounted for the largest revenue of 33.82% in 2023.
- ASOs are short, synthetic strands of nucleotides designed to specifically bind to RNA transcripts and modulate gene expression.
- These therapeutics are widely used in treating genetic disorders such as Duchenne muscular dystrophy and spinal muscular atrophy.
- The increasing approval of ASO-based therapies, advancements in chemical modifications for improved stability and delivery, and their success in addressing unmet clinical needs drive this segment's dominance.
- ASOs dominate the market due to their growing clinical approvals, advancements in stability, and targeted therapeutic potential for genetic disorders.
The small interfering RNAs (siRNAs) segment is anticipated to register the fastest CAGR during the forecast period.
- siRNAs are gaining traction as powerful tools in silencing disease-causing genes by targeting mRNA for degradation.
- The success of RNA interference (RNAi)-based therapies, such as patisiran for hereditary transthyretin amyloidosis, has validated the clinical potential of siRNAs.
- The increasing pipeline of siRNA-based drugs targeting conditions like cancer and infectious diseases further supports their rapid growth.
- siRNAs are expected to grow rapidly, driven by the success of RNAi-based therapies and expanding clinical pipelines targeting diverse diseases.
By Application:
Based on application, the market is segmented into autoimmune disorders, infectious diseases, genetic disorders, and others.
The genetic disorders segment accounted for the largest revenue share of nucleic acid-based therapeutics market share in 2023.
- It offers a promising solution for treating genetic disorders by addressing underlying molecular causes.
- Therapies like ASOs and DNA-based approaches are widely used to correct genetic mutations or modulate gene expression.
- The increasing prevalence of genetic disorders, coupled with advancements in precision medicine and diagnostics, is driving nucleic acid-based therapeutics market demand for these therapies.
- Regulatory approvals for therapies targeting rare genetic conditions further enhance this segment's market position.
- Hence, genetic disorders dominate the market trends, driven by advancements in nucleic acid-based technologies and their ability to address the root cause of genetic conditions.
The autoimmune disorders segment is anticipated to register the fastest CAGR during the forecast period.
- Autoimmune disorders, characterized by immune system dysregulation, are increasingly being targeted by nucleic acid-based therapies that modulate gene expression or immune pathways.
- The development of RNA-based therapies targeting cytokines and immune-regulatory proteins is boosting growth in this segment.
- Rising prevalence of autoimmune conditions like rheumatoid arthritis and multiple sclerosis further supports market expansion.
- Autoimmune disorders analysis is expected to grow rapidly, supported by innovative trends of nucleic acid-based therapies targeting immune dysregulation and inflammatory pathways.
By Delivery Technology:
Based on delivery technology, the market is segmented into Viral Vectors (Retrovirus Vector System, Adeno Virus Vector System, Others) and Non-Viral Vectors (Needle Injection, Electroporation, Others).
The non-viral vector segment accounted for the largest revenue in nucleic acid-based therapeutics market share in 2023.
- Non-viral delivery methods, including needle injection and electroporation, are widely used due to their safety, simplicity, and cost-effectiveness.
- These technologies minimize the risks associated with viral vectors, such as immunogenicity and insertional mutagenesis, making them suitable for clinical applications.
- Electroporation, in particular, is gaining traction for its ability to enhance the delivery of nucleic acid therapies across cell membranes.
- The ongoing advancements in non-viral delivery technologies, including lipid nanoparticles and polymers, further strengthen this segment.
- Non-viral vectors dominate the market trends due to their safety profile, cost-effectiveness, and ongoing advancements in enhancing delivery efficiency.
The viral vector segment is anticipated to register the fastest CAGR during the forecast period.
- Viral vectors, such as retrovirus and adenovirus systems, offer highly efficient delivery of nucleic acids into target cells.
- They are extensively used in gene therapy and cancer immunotherapy due to their high transfection efficiency and ability to target specific cells.
- The increasing use of viral vectors in clinical trials and the development of safer, next-generation viral platforms are driving nucleic acid-based therapeutics market growth in this segment.
- The recent approval of viral vector-based gene therapies also underscores their potential to transform the treatment landscape for genetic and acquired diseases.
- Viral vector analysis is expected to grow rapidly due to their high trends of transfection efficiency and increasing adoption in gene therapy and cancer immunotherapy.
By End-User Industry:
Based on end-user, the market is segmented into pharmaceutical & biotechnology companies, academic & research institutes, contract research organizations (CROs), and hospitals & clinics.
The pharmaceutical & biotechnology companies segment accounted for the largest revenue share in 2023.
- These companies are the primary developers of this therapeutics, leveraging advanced delivery platforms and genetic technologies.
- The increasing focus on precision medicine, coupled with substantial investments in RNA-based therapies and gene editing, drives this segment’s advancement.
- The growing number of clinical trials and regulatory approvals further enhances their market position.
- Pharmaceutical & biotechnology companies dominate the market, driven by their leadership in innovation and product commercialization.
- Hence, the nucleic acid-based therapeutics market analysis depicts the rising trends in advance delivery and efficient therapies that are fueling the market surge.
The contract research organizations (CROs) segment is anticipated to register the fastest CAGR during the forecast period.
- CROs are increasingly favored for preclinical and clinical research outsourcing, particularly in complex areas like nucleic acid-based therapeutics.
- Their expertise in regulatory compliance, trial management, and advanced analytics supports the growing demand for their services.
- The trend of outsourcing to reduce R&D costs and improve efficiency is further fueling acceleration.
- CROs are expected to grow rapidly, supported by the increasing outsourcing of nucleic acid-based therapeutic research and their role in accelerating product development.
- Therefore, the rising nucleic acid-based therapeutics market trends are analysis rapid growth, due to the adoption of new trends in the CROs, advancing their services and reducing R&D is further accelerating the market.
Regional Analysis:
The regions covered are North America, Europe, Asia Pacific, the Middle East and Africa, and Latin America.
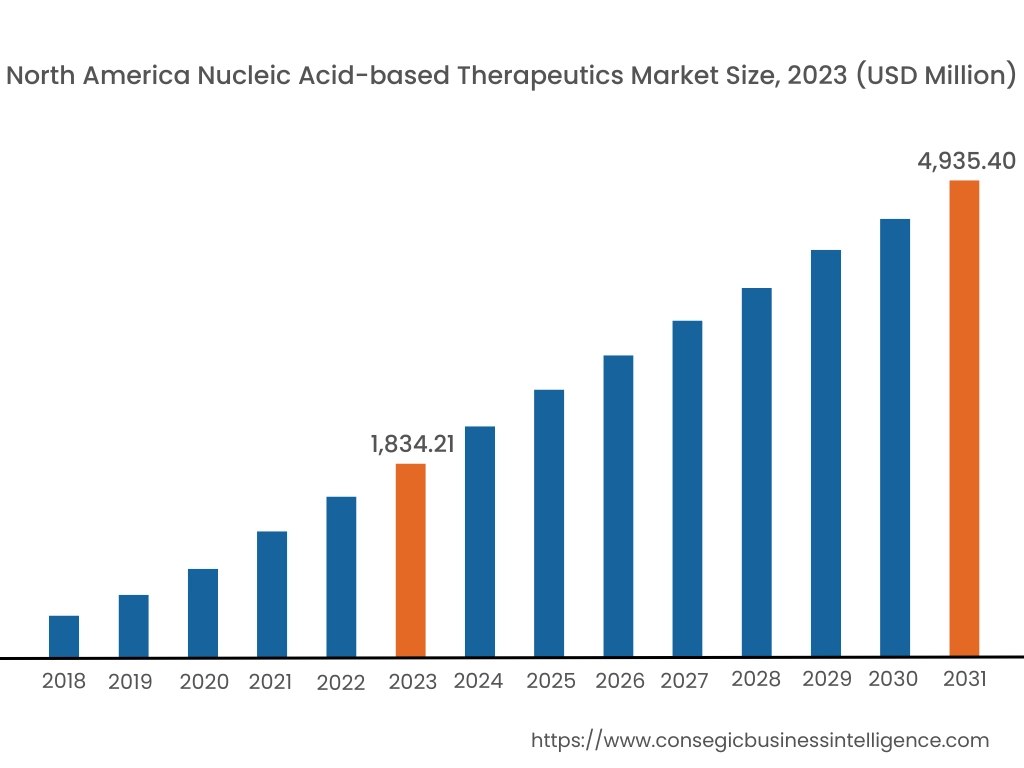
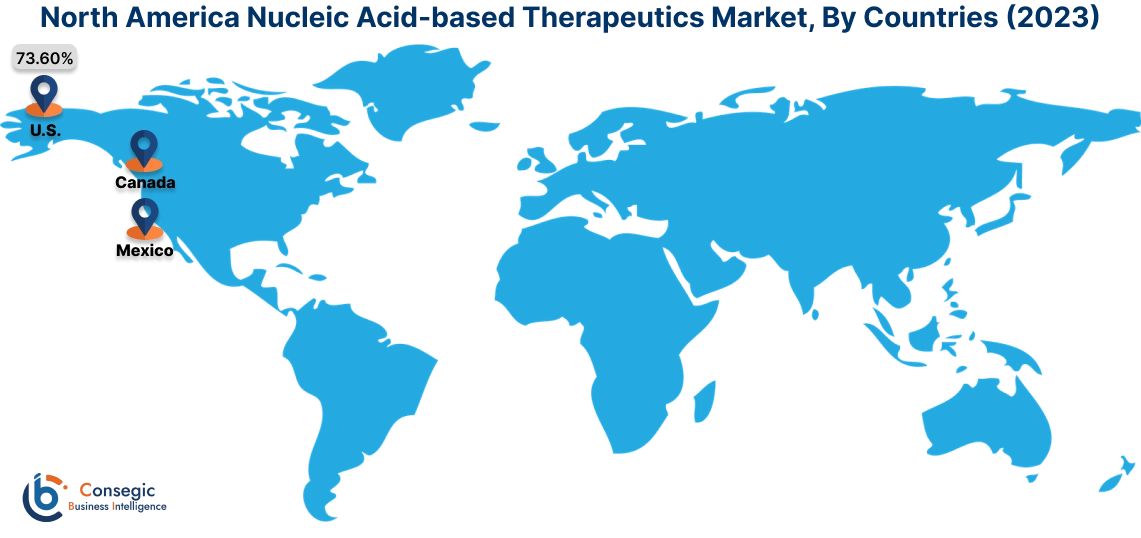
In 2023, North America was valued at USD 1,834.21 Million and is expected to reach USD 4,935.40 Million in 2031. In North America, the U.S. accounted for the highest share of 73.60% during the base year of 2023. North America dominates the nucleic acid-based therapeutics market analysis depicts, it is driven by advanced biotechnology research and robust investments in personalized medicine. The U.S. leads the region with extensive R&D in RNA-based therapies, including mRNA vaccines and antisense oligonucleotides for treating rare and chronic diseases. The presence of major pharmaceutical companies and academic institutions focused on genetic medicine accelerates the market surge. Canada is also contributing through government-backed initiatives promoting genomic research and therapeutic development. However, the high cost of development and stringent regulatory frameworks for genetic therapies can pose challenges for smaller companies in the region.
In Asia Pacific, the market is experiencing the fastest growth with a CAGR of 13.9% over the forecast period. Asia-Pacific is the fastest-growing region in the nucleic acid-based therapeutics market analysis, it is driven by the increasing prevalence of genetic disorders, growing investments in biotechnology, and government support in countries like China, Japan, and South Korea. China is heavily investing in RNA-based therapies and gene editing technologies as part of its healthcare innovation strategy. Japan’s advanced research infrastructure supports the development of nucleic acid-based drugs, particularly for cancer and neurodegenerative diseases. South Korea is focusing on clinical trials and collaborations with global biotech companies to expand its capabilities in genetic medicine. However, challenges such as varying regulatory frameworks and limited access to advanced manufacturing technologies in some parts of the region hinder market nucleic acid-based therapeutics market expansion.
Europe is a significant market for nucleic acid-based therapeutics, supported by strong governmental funding for genomic medicine and advanced healthcare infrastructure. Countries like Germany, the UK, and France are at the forefront, with high adoption rates of therapies targeting genetic disorders and cancers. Germany’s emphasis on RNA-based technologies and the UK’s focus on innovation in gene therapies are driving nucleic acid-based therapeutics market growth. France is seeing increased R&D in nucleic acid-based therapies, particularly for rare diseases. However, the market faces challenges related to lengthy regulatory approval processes and high manufacturing costs for nucleic acid-based drugs.
The Middle East & Africa region is experiencing steady growth in the nucleic acid-based therapeutics industry, driven by increasing healthcare investments and rising demand for advanced treatments for chronic diseases. The UAE and Saudi Arabia are key markets, focusing on modernizing healthcare systems and investing in advanced therapeutics. In South Africa, growing research activities in genomics and the prevalence of infectious diseases like HIV are driving demand for nucleic acid-based solutions. However, the region faces challenges such as limited infrastructure, high treatment costs, and a shortage of skilled professionals in genetic medicine.
Latin America is an emerging market for nucleic acid-based therapeutics, with Brazil and Mexico leading the region. Brazil’s strong focus on advancing genomic research and increasing investment in healthcare industry innovation are driving demand for RNA- and DNA-based therapies. Mexico is also expanding its biotechnology sector, supported by collaborations with international pharmaceutical companies for the development of genetic medicines. However, economic instability, inadequate research infrastructure, and limited public awareness about nucleic acid-based therapies hinder broader adoption in the region.
Top Key Players & Market Share Insights:
The Nucleic Acid-based Therapeutics market is highly competitive with major players providing products and services to the national and international markets. Key players are adopting several strategies in research and development (R&D), product innovation, and end-user launches to hold a strong position in the global Nucleic Acid-based Therapeutics market. Key players in the Nucleic Acid-based Therapeutics industry include -
- Alnylam Pharmaceuticals (United States)
- Ionis Pharmaceuticals (United States)
- Protagonist Therapeutics, Inc. (United States)
- Biogen Inc. (United States)
- Pfizer Inc. (United States)
- Moderna Inc. (United States)
- BioNTech SE (Germany)
- Arrowhead Pharmaceuticals (United States)
- Sarepta Therapeutics (United States)
- Silence Therapeutics plc (United Kingdom)
Recent Industry Developments :
Approvals:
- In December 2023, the FDA approved Casgevy, the first CRISPR-based therapy, for treating sickle cell disease and transfusion-dependent beta-thalassemia. This therapy edits patients' hematopoietic stem cells to produce higher levels of fetal hemoglobin, reducing disease symptoms.
Nucleic Acid-based Therapeutics Market Report Insights :
| Report Attributes | Report Details |
| Study Timeline | 2018-2031 |
| Market Size in 2031 | USD 15,260.97 Million |
| CAGR (2024-2031) | 13.5% |
| By Product Type |
|
| By Application |
|
| By Delivery Technology |
|
| By End-User |
|
| By Region |
|
| Key Players |
|
| North America | U.S. Canada Mexico |
| Europe | U.K. Germany France Spain Italy Russia Benelux Rest of Europe |
| APAC | China South Korea Japan India Australia ASEAN Rest of Asia-Pacific |
| Middle East and Africa | GCC Turkey South Africa Rest of MEA |
| LATAM | Brazil Argentina Chile Rest of LATAM |
| Report Coverage |
|
Key Questions Answered in the Report
What is the size of the nucleic acid-based therapeutics market? +
Nucleic Acid-based Therapeutics Market size is estimated to reach over USD 15,260.97 Million by 2031 from a value of USD 5,530.55 Million in 2023 and is projected to grow by USD 6,180.17 Million in 2024, growing at a CAGR of 13.5% from 2024 to 2031.
What drives the growth of the market? +
Increasing prevalence of genetic disorders, advancements in genomic technologies, and growing investments in personalized medicine.
Which product type holds the largest market share? +
Antisense oligonucleotides (ASOs) lead the market due to clinical approvals, advanced stability, and targeted therapeutic applications.
What are the key applications of nucleic acid-based therapeutics? +
These therapies are used for treating genetic disorders, autoimmune diseases, infectious diseases, and cancer.
Which delivery technology dominates the market? +
Non-viral vectors hold the largest share, offering safety, simplicity, and cost-effectiveness in therapeutic delivery.