- Summary
- Table Of Content
- Methodology
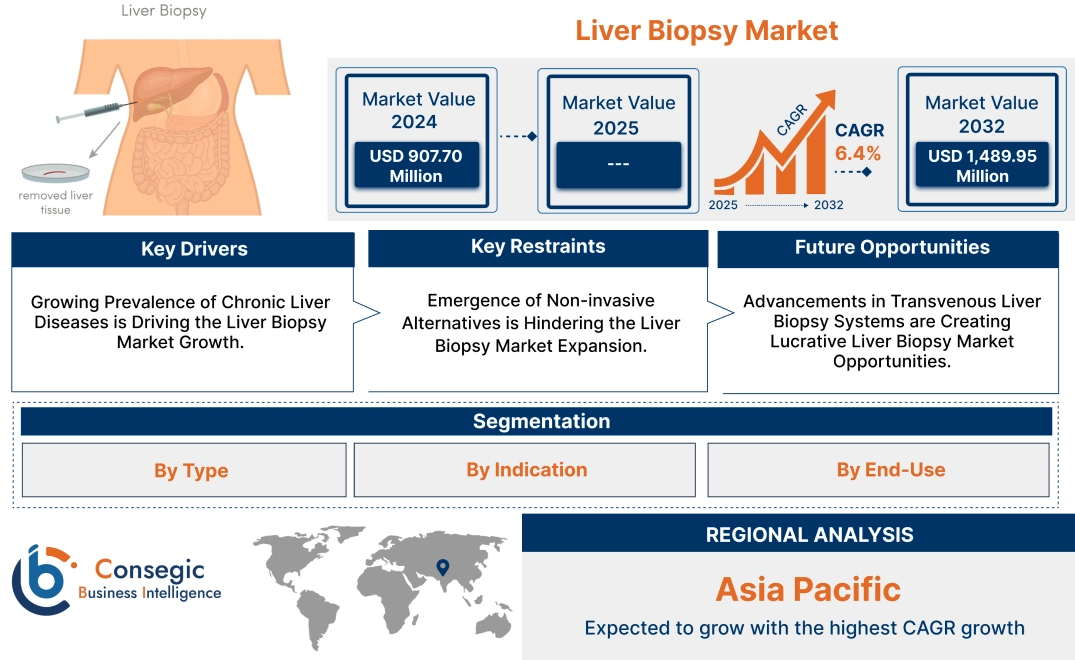
Liver Biopsy Market Size:
Liver Biopsy Market size is growing with a CAGR of 6.4% during the forecast period (2025-2032), and the market is projected to be valued at USD 1,489.95 Million by 2032 from USD 907.70 Million in 2024.
Liver Biopsy Market Scope & Overview:
Liver biopsy is a medical procedure where a small sample of liver tissue is removed for examination under a microscope. This examination helps doctors diagnose liver diseases, assess the severity of liver damage, and monitor the effectiveness of treatment. During biopsy, a thin needle is inserted into the liver, and a small piece of tissue is extracted. The procedure is performed using different techniques, such as percutaneous biopsy, that is performed through the skin, transjugular also known as transvenous biopsy, which is performed through a vein in the neck.
The extracted liver tissue is then examined by a pathologist to identify any abnormalities, such as inflammation, scarring, or the presence of cancer cells. Biopsy results provide crucial information for diagnosing conditions such chronic hepatitis B & C, non-alcoholic fatty liver disease (NAFLD), alcoholic liver disease, liver cirrhosis, and others. The end-user generally includes hospitals, diagnostics centers, ambulatory surgical centers, and others.
Key Drivers:
Growing Prevalence of Chronic Liver Diseases is Driving the Liver Biopsy Market Growth.
The growing prevalence of chronic liver diseases is a significant driver of the liver biopsy market growth. Chronic liver diseases, such as non-alcoholic fatty liver disease (NAFLD), viral hepatitis (B and C), alcoholic liver disease, and autoimmune hepatitis, among others are on the rise globally due to factors like obesity, unhealthy lifestyles, and aging populations. These conditions often progress silently, and biopsy plays a crucial role in their diagnosis and management. By providing tissue samples for histological examination, liver biopsies help assess the extent of liver damage, identify the underlying cause of liver dysfunction, and monitor disease progression. Furthermore, biopsy results are essential for guiding treatment decisions, such as initiating antiviral therapy for hepatitis, initiating treatment for autoimmune hepatitis, or assessing the need for liver transplantation. The increasing prevalence of chronic liver diseases such as hepatitis necessitates a greater number of liver biopsies for accurate diagnosis and effective disease management, thereby driving the growth of the liver biopsy industry.
- According to the data published by the World Health Organization, in 2024, states that, in 2022, 254 million people worldwide were living with hepatitis B, while 50 million were living with hepatitis C. During the same year, there were approximately 2.2 million new infections globally, with 1.2 million attributed to hepatitis B and nearly 1 million attributed to hepatitis C
Hence, these chronic liver diseases often present with subtle or nonspecific symptoms, making biopsy an essential tool for differentiating between various types of liver disease and ruling out other conditions, thus driving the liver biopsy market expansion.
Development of Novel Biopsy Needles is Propelling the Liver Biopsy Market Demand.
The development of novel needles is a key factor propelling the requirement for liver biopsy procedures. Traditional biopsy needles often carry a risk of complications such as bleeding and pain. However, advancements in needle technology have led to the development of innovative devices that enhance safety and improve overall biopsy experience. These advancements include the development of smaller-gauge needles that minimize tissue trauma and reduce the risk of bleeding. Furthermore, the development of flexible needles helps to navigate complex anatomical structures more effectively. Manufacturers are developing novel needle designs that are useful for effective biopsy procedures.
- In 2023, The Boston Scientific developed Acquire™ EUS-FNB 19ga, which is a needle biopsy device which has flexible needle design, particularly well-suited for liver biopsy cases that require enhanced needle flexibility for navigation and tissue acquisition within challenging lobe anatomy. This flexibility allows for greater maneuverability within the liver, enabling physicians to reach and sample target areas more effectively, even in cases with complex anatomical variations.
Thus, these technological advancements are not only improving patient outcomes but also increasing the acceptance and utilization of biopsy procedures among healthcare providers, thereby contributing significantly to the market growth.
Key Restraints:
Emergence of Non-invasive Alternatives is Hindering the Liver Biopsy Market Expansion.
The emergence of non-invasive alternatives is significantly hindering the development of the market. The development and refinement of advanced imaging techniques, such as high-resolution ultrasound, elastography, and magnetic resonance imaging (MRI), alongside the advancement of non-invasive biomarkers for fibrosis, are providing alternative means for assessing liver function and diagnosing certain liver diseases. These non-invasive methods offer the advantage of reduced risk to patients compared to biopsy, which carries inherent risks such as bleeding, infection, and pneumothorax. Manufacturers are introducing non-invasive diagnostic tests for the liver that bypasses the use of biopsy.
- In 2022, Metadeq Inc., a global NASH and metabolic diseases diagnostics company, introduced a novel, non-invasive blood test. This innovative test utilizes two novel circulating proteins to accurately diagnose Non-alcoholic steatohepatitis (NASH) and liver fibrosis, including the ability to stage both diseases. Importantly, this advancement eliminates the need for invasive liver biopsies, a procedure associated with potential risks and discomfort for patients.
Thus, as these non-invasive technologies continue to improve in accuracy and sensitivity, their adoption is likely to increase, potentially reducing the requirement for liver biopsies in certain clinical scenarios.
Future Opportunities :
Advancements in Transvenous Liver Biopsy Systems are Creating Lucrative Liver Biopsy Market Opportunities.
The development of transvenous liver biopsy systems is creating lucrative liver biopsy market opportunities. A transvenous biopsy involves obtaining a liver tissue sample through a vein, typically accessed via the jugular vein in the neck. Advancements in catheter design, imaging guidance, and procedural techniques are enhancing the safety and efficacy of transvenous liver biopsies, making them increasingly attractive to both physicians and patients. Manufacturers are introducing novel systems that offer enhanced safety.
For instance,
- In 2024, Argon Medical's TLAB Transvenous Liver Biopsy System received FDA clearance for transfemoral access, representing a significant advancement in the field. This system incorporates a unique feature that allows physicians to adjust the shape of the device to best suit the patient's individual anatomy. This flexibility enhances navigability and improves sample acquisition in challenging anatomical scenarios, ultimately contributing to the success and safety of the procedure.
This growing adoption of transvenous approaches is driving requirement for specialized equipment, consumables, and training, creating lucrative opportunities for medical device manufacturers and healthcare providers.
Liver Biopsy Market Segmental Analysis :
By Type:
Based on type, the market is categorized into percutaneous, laparoscopic, transvenous.
Trends in the Type:
- Development of flexible needles for better diagnosis is influencing the development of the segment.
- Rise in the trend for minimally invasive procedures, in influencing the segment.
The percutaneous segment accounted for the largest market share in 2024.
- Percutaneous liver biopsy is a minimally invasive procedure used to obtain a small sample of liver tissue for diagnostic purposes.
- It involves inserting a thin needle through the skin and into the liver, typically under the guidance of imaging techniques such as ultrasound, computed tomography (CT), or fluoroscopy.
- The needle is advanced into the liver parenchyma, and a small core of tissue is extracted.
- This tissue sample is then examined under a microscope by a pathologist to identify abnormalities such as inflammation, scarring, fatty infiltration, or the presence of cancer cells.
- Percutaneous liver biopsy is a valuable diagnostic tool for evaluating various liver diseases, including chronic hepatitis, cirrhosis, fatty liver disease, and liver tumors among others.
The transvenous segment is expected to grow at the fastest CAGR over the forecast period.
- Transvenous liver biopsy is a minimally invasive procedure that involves accessing the liver through a vein, typically the jugular vein in the neck.
- This approach differs from percutaneous liver biopsy, where the needle is inserted directly through the abdominal wall.
- By traversing the venous system, transvenous liver biopsy significantly reduces the risk of complications such as pneumothorax, a potential risk associated with percutaneous procedures that involve directly puncturing the liver parenchyma.
- This technique offers several advantages, including improved access to challenging liver segments and a reduced risk of complications, making it a valuable alternative for many patients requiring liver tissue sampling.
- Manufacturers are introducing novel biopsy kits for sampling liver tissues.
- For instance, Cook Medical has designed a specialized Liver Access and Biopsy Set, which is intended for use in medical procedures that involve accessing the liver for diagnostic. This comprehensive set includes various instruments and tools that facilitate safe and effective liver biopsies through the vein present in the neck region, enhancing the accuracy of the procedures while minimizing patient discomfort and risk.
- Thus, these aforementioned factors are influencing the liver biopsy market trends in the coming years.
By Indication:
Based on the indication, the market is categorized into chronic hepatitis B & C, non-alcoholic fatty liver disease (NAFLD), alcoholic liver disease, liver cirrhosis, and others.
Trends in Indication:
- The rise in use of biopsy for the diagnosis of hepatitis is influencing the segment
The non-alcoholic fatty liver disease (NAFLD) segment accounted for the largest market share in the year 2024 and is expected to grow at the fastest CAGR over the forecast period.
- Non-alcoholic fatty liver disease (NAFLD), characterized by the accumulation of excess fat in the liver in the absence of significant alcohol consumption.
- While imaging techniques like ultrasound and elastography provide valuable information, liver biopsy remains crucial for definitively diagnosing NAFLD and differentiating it from other liver diseases.
- Biopsy allows for histopathological evaluation, enabling the assessment of the severity of liver inflammation, the presence of fibrosis (scarring), and the presence of ballooning degeneration.
- This information is critical for guiding treatment decisions, predicting disease progression, and assessing the need for further interventions.
- The indication has become increasingly prevalent globally due to rising rates of obesity and type 2 diabetes.
- For instance, according to the data published by the Indian Journal of Community Medicine, in 2023, the prevalence of Non-alcoholic Fatty Liver Disease (NAFLD) is significantly high globally, with estimates exceeding 25% of the adult population. In India, the prevalence of NAFLD ranges from 9% to 32% of the general population. This high prevalence is closely linked to the rising rates of obesity, type 2 diabetes, and metabolic syndrome, which are increasingly prevalent worldwide.
- Thus, as per the market analysis, the aforementioned factors are influencing the development of the liver biopsy market trends in the coming years.
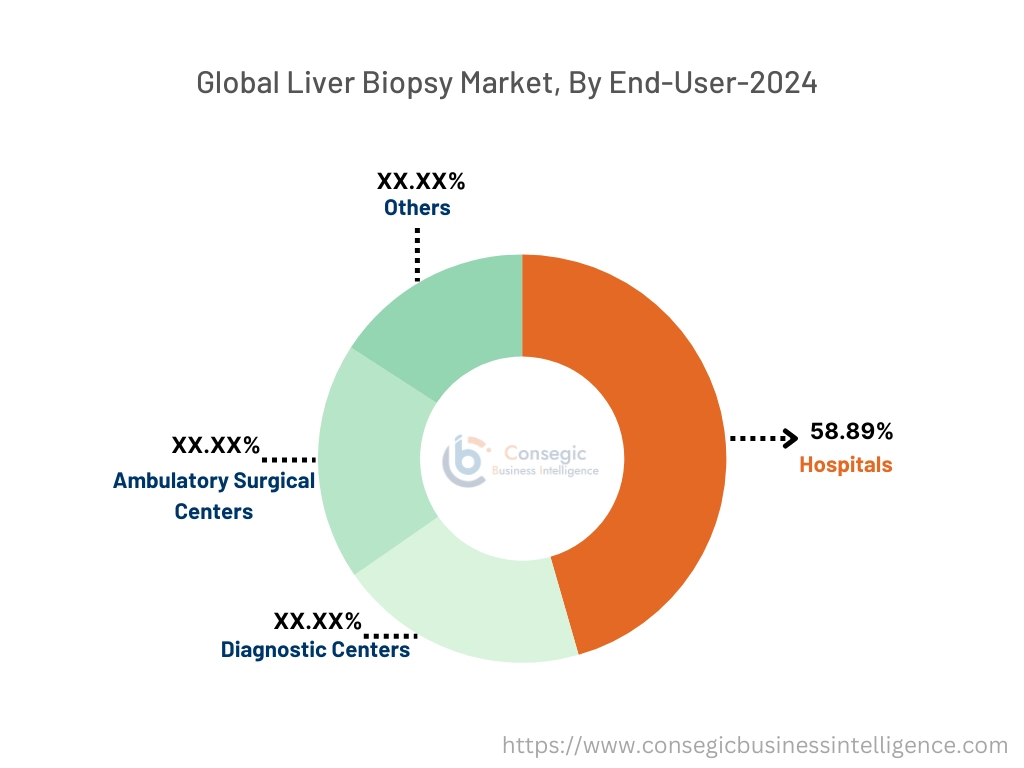
By End-User:
Based on end user, the market is categorized into hospitals, diagnostic centers, ambulatory surgical centers, and others.
Trends in the End User:
- Expansion of diagnostic centers are influencing the segment
- Rise in the trend for same day procedures and discharge is influencing the segment.
The hospitals segment accounted for the largest market share of 58.89% in the year 2024.
- Hospitals play a pivotal role as end-users in the liver biopsy industry. They serve as the primary centers for performing these procedures, housing the necessary infrastructure, equipment, and expertise.
- Hospitals typically have dedicated departments of gastroenterology, hepatology, and interventional radiology, staffed with experienced physicians and trained technicians.
- These departments are equipped with advanced imaging modalities such as ultrasound, computed tomography (CT), and fluoroscopy, which are essential for guiding liver biopsy procedures.
- Furthermore, hospitals provide a comprehensive care setting, enabling the management of potential complications and ensuring patient safety and recovery following the procedure.
- Various hospitals are introducing novel biopsy procedures for proper sampling and early detection of indications.
- For instance, in 2024, Apollo Hospitals, announced the inauguration of its latest endeavor, the Apollo Fatty Liver Clinic at the Liver Diseases and Transplantation Institute, Apollo Hospital. This specialized clinic, equipped with state-of-the-art diagnostic tools like FibroScan for non-invasive assessment of liver fibrosis, will offer comprehensive care for individuals with fatty liver disease. The clinic will focus on early detection, accurate diagnosis, and personalized treatment plans, including lifestyle modifications, medication management, and advanced interventional procedures when necessary. It also includes liver biopsy that is necessary for definitive diagnosis and staging of liver disease. This comprehensive approach will ensure patients receive the most appropriate and effective care for their specific condition.
- Thus, based on the market analysis, these aforementioned factors are influencing the market demand.
The ambulatory surgical centers segment is expected to grow at the fastest CAGR over the forecast period.
- Ambulatory surgical centers (ASCs) are increasingly playing a significant role as end-users in the market.
- These facilities offer a cost-effective and convenient alternative to hospital settings for many minimally invasive procedures, including liver biopsies.
- ASCs are equipped with modern technology and staffed by experienced physicians and nurses, enabling them to perform a range of outpatient procedures safely and efficiently.
- The shift towards outpatient settings for liver biopsies offers several advantages, including reduced healthcare costs, improved patient convenience, and faster recovery times.
- Thus, based on the liver biopsy market analysis, the aforementioned factors are influencing segment growth in the coming years.
Regional Analysis:
The regional segment includes North America, Europe, Asia Pacific, the Middle East and Africa, and Latin America.
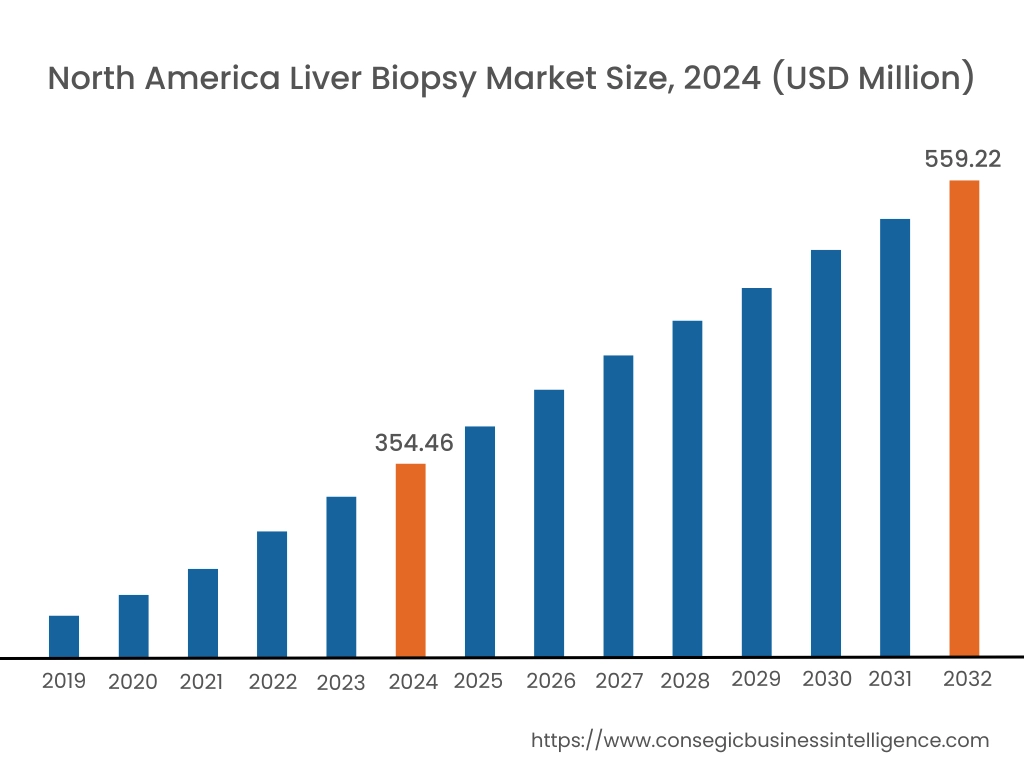
In 2024, North America accounted for the highest market share at 39.05% and was valued at USD 354.46 Million and is expected to reach USD 559.22 Million in 2032. In North America, U.S. accounted for the highest liver biopsy market share of 73.05% during the base year of 2024.
The North American market for liver biopsy is currently dominant, driven by several factors. The region boasts a well-developed healthcare infrastructure with advanced diagnostic capabilities, including sophisticated imaging modalities and access to experienced interventional radiologists. Furthermore, the high prevalence of chronic liver diseases such as non-alcoholic fatty liver disease (NAFLD), hepatitis C, and alcoholic liver disease in the region significantly contributes to the demand for liver biopsies.
For instance,
- According to the data published by the American Liver Foundation, in 2023, states that over 100 million people in the U.S. are estimated to have some form of liver disease, a significant portion of this burden stems from non-alcoholic fatty liver disease (NAFLD). Although 4.5 million U.S. adults have been diagnosed with liver disease, a staggering 80-100 million adults are believed to have NAFLD
Additionally, the presence of key market players and robust research and development activities within the medical device and diagnostics sectors further fuel market development in North America.
Asia Pacific is experiencing the fastest growth with a CAGR of 7.2% over the forecast period. The Asia-Pacific region represents a vibrant and rapidly evolving market for liver biopsy procedures, influenced by a variety of interconnected factors. Firstly, the region is experiencing a substantial increase in population, accompanied by a growing prevalence of chronic liver diseases. These conditions include viral hepatitis, which remains a significant public health concern, non-alcoholic fatty liver disease (NAFLD), which is on the rise due to lifestyle factors, and alcoholic liver disease, often linked to increasing alcohol consumption. This alarming trend necessitates a heightened demand for effective diagnostic tools such as biopsy, which is crucial in determining appropriate treatment strategies and in monitoring the progression of these diseases over time. Countries within the Asia-Pacific are investing significantly in their healthcare infrastructure, leading to improvements in medical services and accessibility for patients. Based on the market analysis, enhanced healthcare facilities and services are facilitating easier access to diagnostic and treatment options for liver diseases, thus driving the market.
Europe presents a significant contribution to the Liver Biopsy market analysis. The European market for liver biopsy is a mature market characterized by well-developed healthcare systems and a strong focus on patient-centered care. Europe boasts a high prevalence of chronic liver diseases, including viral hepatitis, alcoholic liver disease, and non-alcoholic fatty liver disease, driving the demand for diagnostic procedures like liver biopsy. The region has a well-established network of hospitals and clinics with access to advanced imaging technologies and experienced interventional radiologists. Furthermore, the presence of robust regulatory frameworks and a focus on research and development within the medical device sector contribute to the market in Europe. However, factors such as varying healthcare expenditure across different European countries and the increasing adoption of non-invasive diagnostic modalities influence the growth trajectory of the market.
The Middle East and Africa (MEA) region is witnessing notable Liver Biopsy market demand characterized by significant potential. The market in the Middle East and Africa regions present a diverse landscape. While some countries within these regions boast advanced healthcare infrastructure and access to modern diagnostic techniques, others face significant challenges in terms of healthcare access and resource availability. The increasing prevalence of chronic liver diseases, particularly viral hepatitis, driven by factors like limited access to vaccination and treatment, is a significant driver for the requirement for liver biopsies in this region. However, the market faces several challenges, including limited access to specialized healthcare facilities and skilled personnel, inadequate healthcare infrastructure in some regions, and economic limitations that restrict access to diagnostic procedures for a significant portion of the population. Despite these challenges, there is significant potential for market development in the region. Based on the market analysis, investments in improving healthcare infrastructure, expanding access to diagnostic services, and raising awareness about liver diseases are crucial for enhancing the utilization of liver biopsy and improving patient outcomes in the Middle East and Africa.
Latin America is an emerging region in the Liver Biopsy market share, with significant potential for innovation. The market in Latin America is characterized by a diverse landscape with significant growth potential. While some countries within the region have well-developed healthcare systems, others face challenges in terms of access to quality healthcare and limited resources. The increasing prevalence of chronic liver diseases, including viral hepatitis, non-alcoholic fatty liver disease (NAFLD), and alcohol-related liver disease, is driving the requirement for liver biopsies. However, market development is influenced by factors such as economic disparities, varying levels of healthcare infrastructure across different countries, and limited access to advanced diagnostic technologies in certain regions. The development of more affordable and accessible diagnostic options, along with investments in improving healthcare infrastructure and raising awareness about liver diseases, are crucial for driving market development and improving patient outcomes in the Latin American region.
Top Key Players and Market Share Insights:
The global Liver Biopsy market is highly competitive with major players providing precise products to the national and international markets. Key players are adopting several strategies in research and development (R&D) and product innovation to hold a strong position in the global Liver Biopsy market. Key players in the Liver Biopsy industry include-
- Boston Scientific Corporation (United States)
- Argon Medical Devices (United States)
- Braun (Germany)
- Cardinal Health (United States)
- Medtronic (United States)
- Cook (United States)
- Advin Health Care (India)
- Becton, Dickinson, and Company (United States)
- FUJIFILM (Japan)
- Terumo India Pvt Ltd, (India)
Recent Industry Developments :
Product Launch:
- In 2024, Argon Medical's TLAB Transvenous Liver Biopsy System received FDA clearance for transfemoral access, representing a significant advancement in the field. This system incorporates a unique feature that allows physicians to adjust the shape of the device to best suit the patient's individual anatomy. This flexibility enhances navigability and improves sample acquisition in challenging anatomical scenarios, ultimately contributing to the success and safety of the procedure.
Liver Biopsy Market Report Insights :
| Report Attributes | Report Details |
| Study Timeline | 2019-2032 |
| Market Size in 2032 | USD 1,489.95 Million |
| CAGR (2025-2032) | 6.4% |
| By Type |
|
| By Indication |
|
| By End Use |
|
| By Region |
|
| Key Players |
|
| North America | U.S. Canada Mexico |
| Europe | U.K. Germany France Spain Italy Russia Benelux Rest of Europe |
| APAC | China South Korea Japan India Australia ASEAN Rest of Asia-Pacific |
| Middle East and Africa | GCC Turkey South Africa Rest of MEA |
| LATAM | Brazil Argentina Chile Rest of LATAM |
| Report Coverage |
|
Key Questions Answered in the Report
How big is the Liver Biopsy market? +
In 2024, the Liver Biopsy market is USD 907.70 Million.
Which is the fastest-growing region in the Liver Biopsy market? +
Asia Pacific is the fastest-growing region in the Liver Biopsy market.
What specific segmentation details are covered in the Liver Biopsy market? +
Type, Indication, and End User segmentation details are covered in the Liver Biopsy market.
Who are the major players in the Liver Biopsy market? +
Boston Scientific Corporation (United States), Argon Medical Devices (United States), and Cook (United States) are some of the major players in the market.