- Summary
- Table Of Content
- Methodology
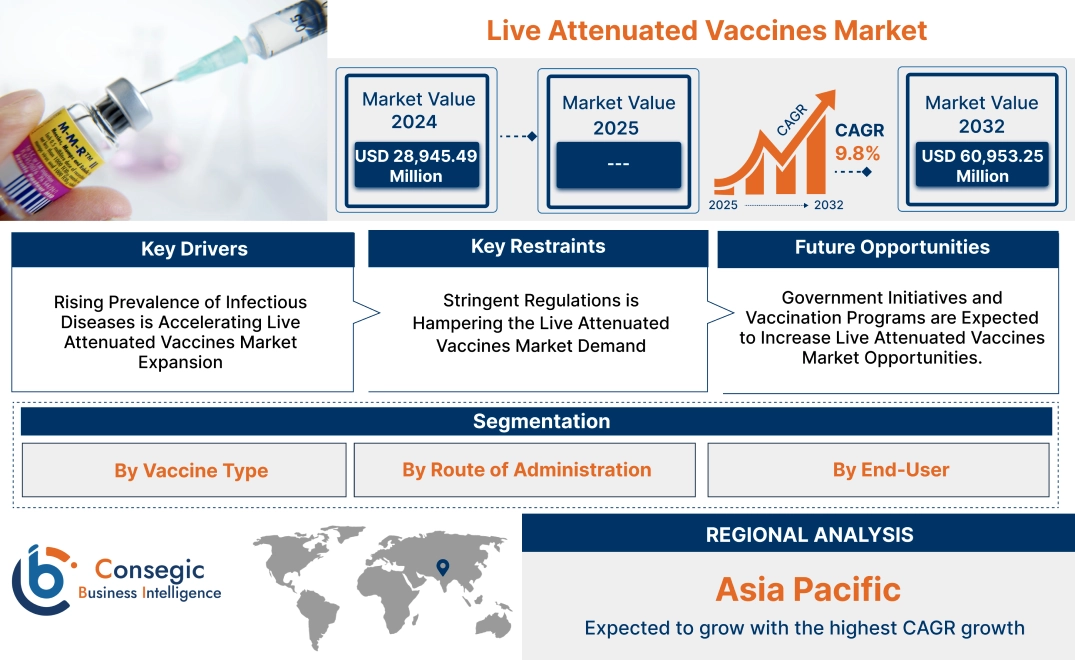
Live Attenuated Vaccines Market Size:
Live Attenuated Vaccines Market size is growing with a CAGR of 9.8% during the forecast period (2025-2032), and the market is projected to be valued at USD 60,953.25 Million by 2032 from USD 28,945.49 Million in 2024.
Live Attenuated Vaccines Market Scope & Overview:
Live attenuated vaccines (LAV) are a type of vaccine that uses a weakened form of a living virus or bacterium to stimulate an immune response. These vaccines are highly effective in preventing various diseases, including measles, mumps, rubella, chickenpox, polio, and tuberculosis. These vaccines are created by modifying the pathogen in a laboratory to make it less virulent. This is done through various methods, such as culturing the pathogen in unusual conditions or genetically altering it. The weakened pathogen is then introduced into the body, where it replicates to a limited extent, triggering an immune response. These vaccines create a strong and long-lasting immune response because they are like the natural infection they prevent. One or two doses of a live vaccine provide a lifetime of protection against a disease.
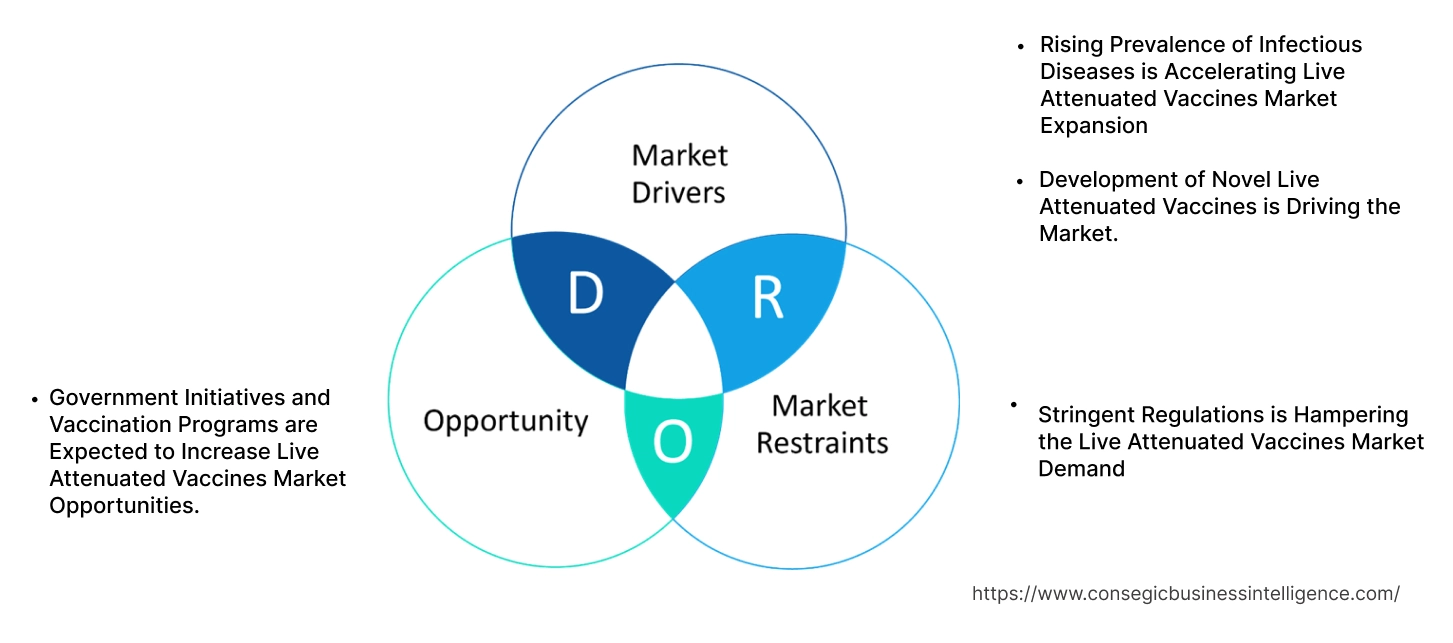
Key Drivers:
Rising Prevalence of Infectious Diseases is Accelerating Live Attenuated Vaccines Market Expansion
An infectious disease is a disorder caused by an organism such as a bacterium, virus, parasite, or fungus transmitted to the human body. Symptoms range from mild to life-threatening and vary depending on the organism causing the infection. Common symptoms include fever, fatigue, chills, and others.
Live attenuated vaccines offer several significant benefits in the fight against infectious diseases. They provide long-lasting immunity with just one or two doses, eliminating the need for frequent booster shots. Moreover, these vaccines are particularly effective in stimulating mucosal immunity, which is crucial for protection against infections that enter the body through mucosal surfaces such as the respiratory tract or the gastrointestinal tract. Increased global travel and trade and weakened public health systems have led to a rise in cases of infectious diseases.
- According to Florida Health, varicella (Chickenpox) cases increased by 87.64% from 348 in 2020 to 653 in 2023. This has led to an increased need for live attenuated vaccines to treat it, positively influencing live attenuated vaccines market trends.
Overall, the rising prevalence of infectious diseases is significantly boosting the live attenuated vaccines market expansion.
Development of Novel Live Attenuated Vaccines is Driving the Market.
The emergence of new infectious diseases, such as COVID-19 and Ebola, highlights the urgent need for effective vaccines. Moreover, the evolving nature of existing infectious diseases, such as influenza and HIV, necessitates the development of new and improved vaccines to maintain effective disease control. Research and development efforts of manufacturers are focused on creating novel vaccines for a wide range of diseases, neglected tropical diseases, and diseases with limited or no effective vaccine options.
- In 2023, Valneva successfully gained FDA approval for Ixchiq, marking the first-ever chikungunya vaccine. It contains a lively, weakened version of the Chikungunya virus and is intended for adults aged 18 and above who face a heightened risk of contracting the Chikungunya virus. These new launches are positively influencing the live attenuated vaccines market trends.
Moreover, new R&D efforts are being taken involving innovative approaches such as genetic engineering, synthetic biology, and novel delivery systems to enhance vaccine efficacy, safety, and stability.
Overall, the development of new novel vaccines is accelerating the global live attenuated vaccines market growth.
Key Restraints:
Stringent Regulations is Hampering the Live Attenuated Vaccines Market Demand
Regulatory hurdles are one of the significant barriers to the global live attenuated vaccines market growth. These vaccines are subject to strict regulations by authorities such as the U.S. FDA, the European Medicines Agency (EMA), and other national regulatory bodies. These regulations ensure the safety, efficacy, and quality of products, but this process is time-consuming and expensive, requiring significant investment from vaccine manufacturers.
The approval process for these vaccines is lengthy and costly. Manufacturers must conduct extensive trials, including preclinical studies, phase 1, 2, and 3 clinical trials which demonstrate compliance with safety standards of their products before they receive approval. This process not only delays the availability but also increases the cost of development, which is ultimately passed on to consumers and healthcare providers.
Furthermore, variations in regulatory standards across different regions further complicate market entry for companies. What is approved in one country takes additional time in another, making it difficult for global companies to maintain a seamless product launch strategy.
Overall, analysis shows that the stringent regulatory requirements along with lengthy approval processes and varying regulatory standards across different regions are hampering the live attenuated vaccines market demand.
Future Opportunities :
Government Initiatives and Vaccination Programs are Expected to Increase Live Attenuated Vaccines Market Opportunities.
Vaccination programs are essential for preventing the spread of infectious diseases and protecting individuals and communities from outbreaks. High vaccination rates contribute to herd immunity, which protects vulnerable individuals who cannot be vaccinated (e.g., infants, and immunocompromised individuals). Moreover, the reduced disease burden leads to lower healthcare costs, increased productivity, and improved economic outcomes. Climatic change leading to rapid pathogen dissemination and antimicrobial resistance limiting the effectiveness of treatments have to government to take serious initiatives to ensure that all eligible individuals receive recommended vaccines ensuring that more individuals are protected from preventable diseases.
- In October 2024, the NHS in Leicester initiated a free vaccination program for the upcoming autumn and winter seasons, covering both COVID-19 and influenza. This will boost the demand for vaccines, creating the potential for market growth.
Additionally, governments are making targeted efforts to vaccinate individuals who missed doses in the past. Furthermore, they are reaching underserved populations, such as those living in remote areas or experiencing homelessness. Apart from all these trends, there are campaigns aiming to educate the public about the importance of vaccination, address vaccine hesitancy, and promote vaccine uptake.
Overall, government-led vaccination programs are expected to increase live attenuated vaccines market opportunities.
Live Attenuated Vaccines Market Segmental Analysis :
By Vaccine Type:
Based on vaccine type, the market is categorized into viral and bacterial.
Trends in Vaccine Type:
- Growing focus on developing novel LAV, particularly for emerging infectious diseases.
- Bacterial LAV is gaining momentum, driven by the increasing prevalence of bacterial infections.
The viral segment accounted for the largest market share in 2024
- Viral vaccine type is further categorized into measles-mumps-rubella (MMR), influenza, polio, and others
- The viral segment dominates due to the high prevalence of viral diseases. Diseases caused by viruses to humans include measles, mumps, rubella, polio, influenza, and many others. These diseases pose significant public health challenges globally, driving the need for effective preventive measures such as live vaccines.
- Moreover, LAV has a successful history in preventing numerous viral diseases such as influenza, mumps, rubella, and polio amongst others. It is more effective in comparison to other vaccines.
- For instance, according to an article published by Consensus, in 2024, inactivated measles vaccines have been shown to provide shorter-lasting antibodies compared to live vaccines while demonstrating better child survival rates.
- Additionally, significant research and development efforts have been invested in developing and improving live vaccines against various viral diseases. This has led to the development of highly effective and safe vaccines for many common viral infections, driving segment.
- Increased public awareness about the importance of vaccination and its role in disease prevention drives the segment.
- Overall, as per the market analysis, the high prevalence of viral diseases, their proven efficacy, and ongoing research and development, coupled with increased public awareness, are driving the segment in the global live attenuated vaccines market growth.
The bacterial segment is expected to grow at the fastest CAGR over the forecast period.
- Bacterial vaccine type of further categorized into bacillus calmette-guérin (BCG), typhoid, cholera, and others
- Despite advancements in medicine, bacterial infections continue to pose a significant public health threat. The rise of antibiotic resistance, coupled with the emergence of new bacterial strains, has made these infections more difficult to treat. This heightened concern necessitates the development of effective preventive measures such as live vaccines.
- Moreover, tuberculosis remains a major global health concern, with millions of new infections reported annually. The BCG vaccine, while partially effective, has limitations in terms of efficacy and duration of protection. Ongoing research and development efforts to improve the efficacy and broaden the coverage of the BCG vaccine, as well as the development of novel tuberculosis vaccines, are driving significant market in this area.
- Additionally, research and development efforts are actively exploring the development of live vaccines for other bacterial infections, such as typhoid and cholera. These diseases continue to pose significant public health challenges, particularly in developing countries.
- According to market analysis, successful development and commercialization of LAV for these diseases would significantly expand the market for the bacterial segment in the upcoming years.
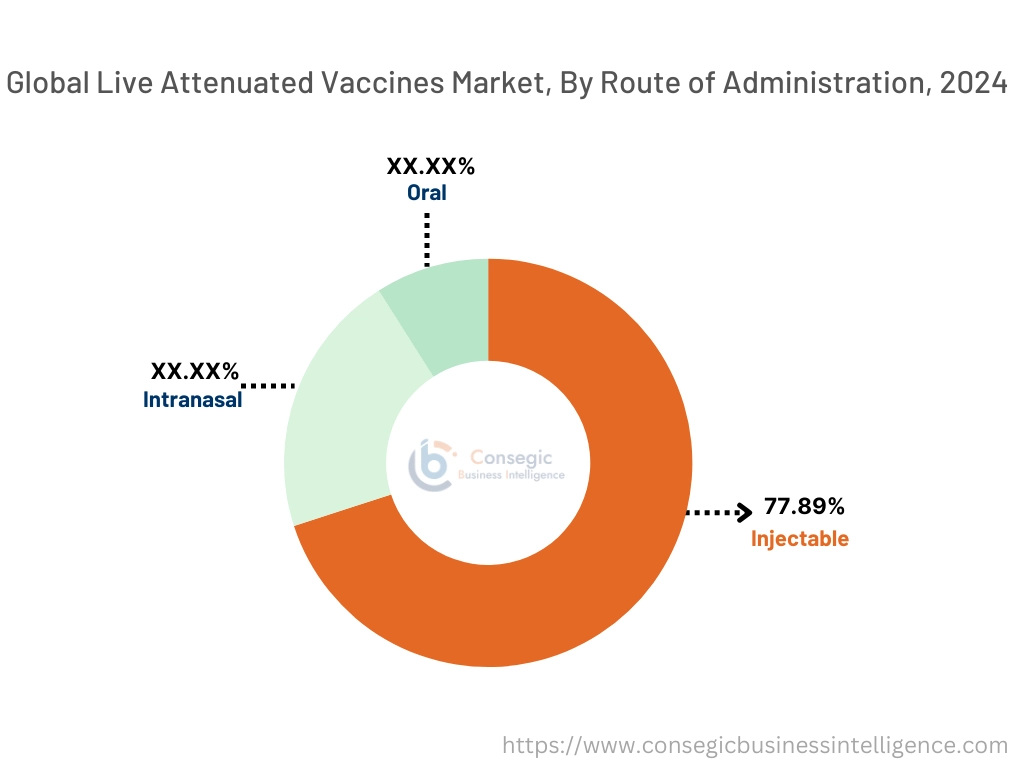
By Route of Administration:
The route of the administration segment is categorized into oral, injectable, and intranasal.
Trends in the Route of Administration
- Intramuscular injections are the most common injectable route, and subcutaneous injections are gaining popularity.
- Intranasal vaccines hold significant promise for preventing respiratory infections as it induces a more robust mucosal immune response.
The injectable segment accounted for the largest market share of 77.89% in 2024.
- Injectable vaccines are typically more stable and maintain their potency for longer periods. This is crucial for ensuring consistent efficacy and minimizing wastage during transportation and storage, especially in regions with limited cold chain infrastructure.
- Moreover, injectable routes allow for precise dosing and controlled delivery of the vaccine directly into the bloodstream or muscle tissue. This ensures that the intended amount of the vaccine reaches the target site and elicits a robust immune response.
- Additionally, injectable formulations are less susceptible to contamination from environmental factors or the gastrointestinal tract, which potentially inactivate or degrade the vaccine.
- Furthermore, a well-developed healthcare infrastructure for administering injectable vaccines already exists globally. This includes trained healthcare professionals, readily available syringes and needles, and established vaccination centers.
- As per the market analysis, supportive government policies, including subsidies and tax incentives, are encouraging vaccine development and increasing access to vaccines driving this segmental growth in the live attenuated vaccines industry.
The intranasal segment is expected to grow at the fastest CAGR over the forecast period.
- Intranasal delivery of these vaccines is gaining significant traction.Intranasal administration directly stimulates mucosal immune responses in the respiratory tract, which is the primary entry point for many respiratory infections. This induces the production of mucosal IgA antibodies, providing crucial protection against pathogens.
- Moreover, many individuals find intranasal administration more convenient and less painful than injections, leading to improved patient acceptance and compliance with vaccination schedules.
- Additionally, advancements in delivery systems are enhancing the efficacy and stability of intranasal vaccines, further driving their development and adoption.
- Manufacturers are researching and launching intranasal vaccines against many diseases, with promising results observed in clinical trials. These vaccines aim to provide broader protection by immune responses., driving segment.
- For instance, In 2024, the FDA approved FluMist, a nasal spray flu vaccine. This vaccine contains weakened forms of live influenza viruses and is sprayed into the nose to help prevent the flu.
- Overall, strong research and development activities, coupled with new product launches, will drive the segment for the upcoming years.
By End-User:
The end-user segment is categorized into hospitals, clinics, vaccination centers, and others.
Trends in End-User
- Increasing role of community health centers and pharmacies in vaccine delivery.
- Focus on improving access to vaccination services in underserved communities.
The hospitals segment accounted for the largest market share in 2024.
- Hospitals are equipped with the necessary infrastructure, including cold storage facilities, trained medical personnel, and emergency response capabilities, to safely handle and administer live vaccines.
- Moreover, hospitals serve as central healthcare hubs, providing a wide range of medical services, including vaccinations. This centralized approach facilitates efficient vaccine distribution and administration.
- Additionally, hospitals provide specialized care for vulnerable populations, such as infants, immunocompromised individuals, and the elderly, who require specific monitoring and support after receiving live vaccines.
- The rising healthcare industry in terms of increased investment, technological advancements, growing diseases, and government regulations has led to increases in the number of hospitals.
- For instance, according toCEIC, the number of hospitals in China increased by 3.85% from 2022 to 2023, rising from 36 million units to 38 million units. Consequently, this surge in hospitals has contributed to a higher volume of vaccines to administer to infants, children, and adults, positively driving the segment in the market.
- Overall, robust infrastructure, a central role in healthcare, specialized care capabilities, and increasing numbers are driving the segment.
The vaccination centers segment is expected to grow at the fastest CAGR over the forecast period.
- Vaccination centers are emerging as a prominent end-user segment in the market due to several key trends. Their dedicated focus on vaccinations allows for streamlined processes and improved efficiency. By specializing in this single service, these centers optimize their operations, minimizing wait times and maximizing patient throughput.
- This efficiency translates to a more convenient experience for patients, making vaccinations accessible and less time-consuming.
- Moreover, the increasing need for vaccinations across various populations is driving the need for these specialized centers. Rising awareness about the importance of immunization has improved vaccination rates, significantly increasing the need for vaccination services.
- The government increasing its funding and efforts for the opening of new vaccination centers to ensure more population in the country is protected from diseases will drive the segment for the forecasted years.
Regional Analysis:
The regional segment includes North America, Europe, Asia Pacific, the Middle East and Africa, and Latin America.
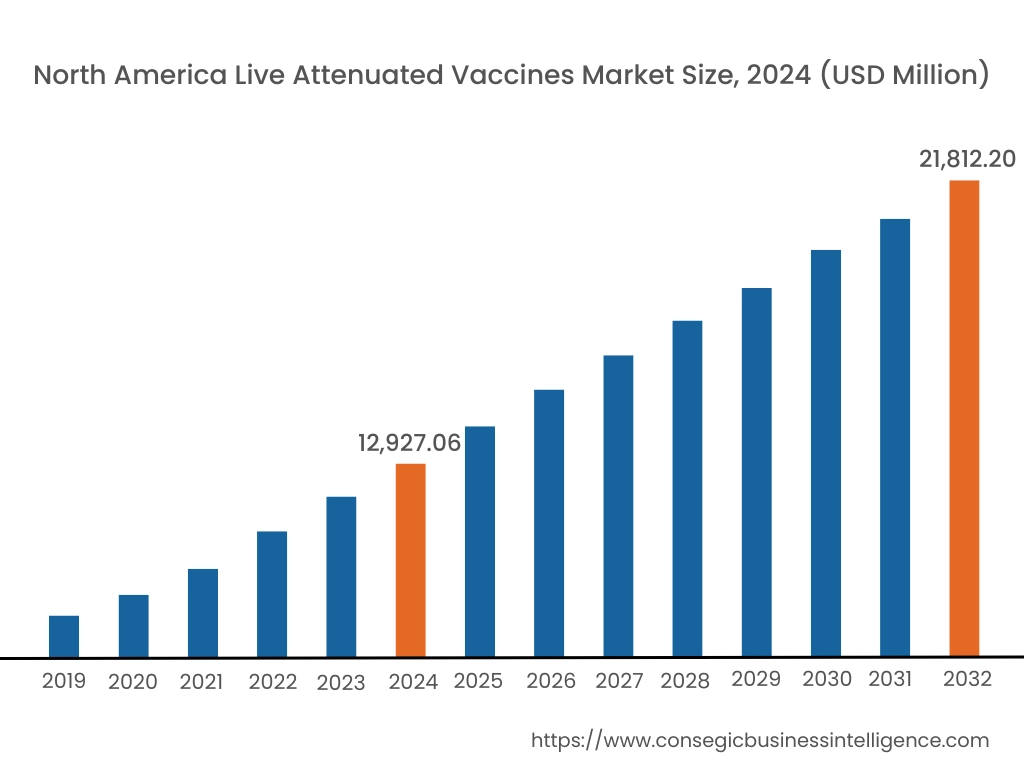
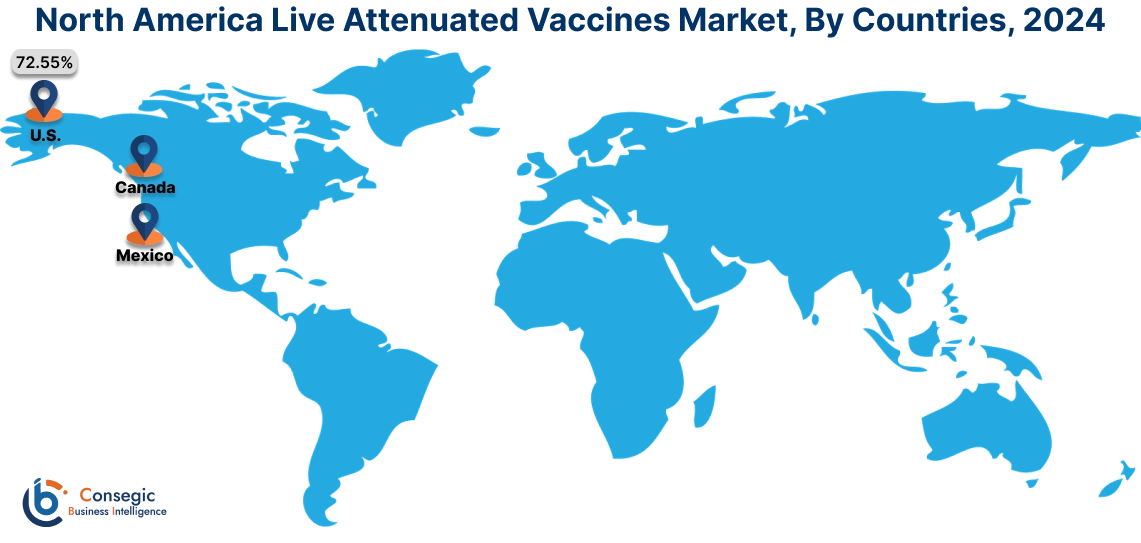
In 2024, North America accounted for the highest live attenuated vaccines market share at 44.66% and was valued at USD 12,927.06 Million and is expected to reach USD 21,812.20 Million in 2032. In North America, the U.S. accounted for the highest live attenuated vaccines market share of 72.55% during the base year of 2024. The region boasts a robust and well-developed healthcare infrastructure, with advanced research facilities and a strong pharmaceutical industry. This fosters a conducive environment for vaccine development, manufacturing, and distribution. Moreover, the investment and funding to increase the availability of vaccines in the nation is further boosting the market.
- For instance, in 2021, the S. Government announced an investment of USD 10 billion to improve access to COVID-19 vaccines and foster trust in vaccination within communities most severely impacted and at the highest risk.
Additionally, North America has a high level of awareness regarding the importance of vaccination and strong government support for immunization programs. This translates into high vaccination rates and a significant demand for vaccines across various age groups. Furthermore, substantial investments in research and development within the region drive innovation in vaccine technology and the development of new and improved live vaccines. Overall, North America dominates the market due to a robust healthcare system, significant government investment in vaccine access and R&D, high public awareness, and strong government support for immunization programs.
In Asia Pacific, the live attenuated vaccines market is experiencing the fastest growth with a CAGR of 8.3% over the forecast period. The region harbors a vast and rapidly expanding population, particularly within countries such as India and China. This demographic presents a significant demand for routine childhood vaccinations to protect against a range of infectious diseases. Moreover, the Asia-Pacific region faces a diverse spectrum of infectious disease challenges, including the resurgence of vaccine-preventable diseases such as measles and pertussis, alongside the emergence of new and re-emerging infectious threats. This necessitates robust immunization programs to safeguard public health. Additionally, rising disposable incomes and increased healthcare expenditure in many Asian countries are enabling greater access to vaccinations. The burgeoning middle class is increasingly seeking preventive healthcare measures, including vaccinations, contributing to the market.
Europe's live attenuated vaccines market analysis states that several trends are responsible for the progress of the market in the region. Europe has a long-standing history of robust government-funded immunization programs. These programs ensure high vaccination coverage across various age groups, driving a significant need for vaccines. For example, the European Union's immunization program, which aims to improve vaccine uptake and prevent vaccine-preventable diseases, has played a crucial role in driving market growth. Moreover, the region boasts a highly developed healthcare infrastructure with advanced medical facilities, a strong network of hospitals and clinics, and a well-established system for vaccine distribution and administration. Additionally, numerous pharmaceutical companies in the region are actively involved in the development and manufacturing of innovative vaccines, contributing to a robust supply chain and fostering advancements in vaccine technology.
The Middle East and Africa (MEA) live attenuated vaccines market analysis states that the region is also witnessing a notable surge. Several countries in the MEA region are experiencing rapid economic increases, leading to increased disposable incomes and improved access to healthcare services. This has boosted the demand for vaccines for various diseases, positively influencing the market. Moreover, the region faces challenges from various infectious diseases, including polio, measles, and hepatitis. This necessitates robust vaccination programs to control outbreaks and protect vulnerable populations. Vaccine hesitancy remains a concern in some parts of the region, driven by factors such as misinformation, lack of trust in healthcare systems, and religious or cultural beliefs. Significant disparities in healthcare access and quality exist across the MEA region.
Latin America's live attenuated vaccines market size is also emerging. Governments across Latin America are prioritizing public health initiatives and investing in robust immunization programs. These programs aim to increase vaccination coverage rates, particularly among vulnerable populations, and improve access to quality healthcare services. The region faces challenges from various endemic diseases, such as dengue, Zika, and Chagas disease. This necessitates robust vaccination programs and ongoing research and development of effective vaccines against these diseases. A burgeoning middle class with increasing disposable income is a significant driver. This growing economic power translates into greater demand for healthcare services, including preventive measures such as vaccinations. Rural populations, marginalized communities, and those living in remote areas face limited access to vaccination services.
Top Key Players and Market Share Insights:
The Live Attenuated Vaccines market is highly competitive with major players providing products to the national and international markets. Key players are adopting several strategies in research and development (R&D) and product innovation to hold a strong position in the global Live Attenuated Vaccines market. Key players in The Live Attenuated Vaccines industry include-
- GlaxoSmithKline (UK)
- Merck & Co., Inc. (U.S.)
- AstraZeneca (UK)
- Emergent BioSolutions (U.S.)
- Tonix Pharmaceuticals (U.S.)
- Sanofi (France)
- Pfizer (U.S.)
- MedImmune, LLC (U.S.)
- Meissa Vaccines (U.S.)
- Novavax (U.S.)
Recent Industry Developments :
Product Launch:
- FDA approved AstraZeneca's self-administered vaccine FluMist. It is a live attenuated influenza vaccine (LAIV), which is administered as a nasal spray for the prevention of influenza.
Live Attenuated Vaccines Market Report Insights :
| Report Attributes | Report Details |
| Study Timeline | 2019-2032 |
| Market Size in 2032 | USD 60,953.25 Million |
| CAGR (2025-2032) | 9.8% |
| By Vaccine Type |
|
| By Route of Administration |
|
| By End User |
|
| By Region |
|
| Key Players |
|
| North America | U.S. Canada Mexico |
| Europe | U.K. Germany France Spain Italy Russia Benelux Rest of Europe |
| APAC | China South Korea Japan India Australia ASEAN Rest of Asia-Pacific |
| Middle East and Africa | GCC Turkey South Africa Rest of MEA |
| LATAM | Brazil Argentina Chile Rest of LATAM |
| Report Coverage |
|
Key Questions Answered in the Report
How big is the Live Attenuated Vaccines market? +
In 2024, the Live Attenuated Vaccines market is USD 60,953.25 Million.
Which is the fastest-growing region in the Live Attenuated Vaccines market? +
Asia Pacific is the fastest-growing region in the Live Attenuated Vaccines market.
What specific segmentation details are covered in the Live Attenuated Vaccines market? +
Route of Administration and End-User segmentation details are covered in the Live Attenuated Vaccines market
Who are the major players in the Live Attenuated Vaccines market? +
GlaxoSmithKline (UK), Merck & Co., Inc. (U.S.), Sanofi (France), Pfizer (U.S.), MedImmune, LLC (U.S.), Meissa Vaccines (U.S.), Novavax (U.S.), AstraZeneca (UK), Emergent BioSolutions (U.S.), and Tonix Pharmaceuticals (U.S.).