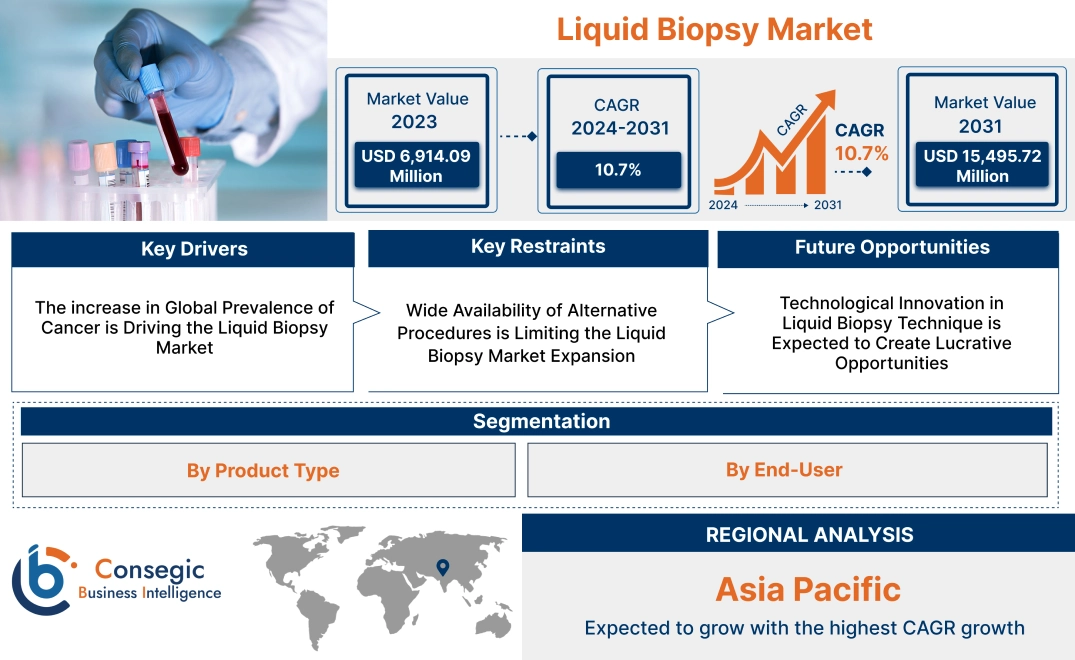
Liquid Biopsy Market Size:
Liquid Biopsy Market size is growing with a CAGR of 10.7% during the forecast period (2024-2031), and the market is projected to be valued at USD 15,495.72 Million in 2031 from USD 6,914.09 Million in 2023.
Liquid Biopsy Market Scope & Overview:
A liquid biopsy is a non-invasive test that analyzes blood, urine, or other body fluids to detect cancer cells or cancer-related DNA fragments. As tumors grow, they release tiny pieces of themselves into the bloodstream. These circulating tumor cells (CTCs) or circulating tumor DNA (ctDNA) can be captured and examined in a lab. This provides valuable information about the cancer, such as its type, stage, and potential response to different treatments. This technique involves the genomics and proteomic assessment of tumor cells. It is widely used in early screening of tumors, perioperative management, and other aspects of cancer detection.
This procedure is increasingly being opted for the diagnosis of lung, breast, and prostate cancers, amongst others. One of the significant advantages of this technique is that it is much faster than a surgical biopsy. Additionally, early detection of disease progression is done using this technique. This method focuses on different types of biomarker detection such as circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and exosomes.
How is AI Transforming the Liquid Biopsy Market?
AI is increasingly being used in the liquid biopsy market, particularly for analyzing complex blood sample data to improve early cancer detection, accuracy, and personalized treatment. AI-powered systems can analyze complex liquid biopsy data to identify novel biomarkers for early cancer detection, improve tumor characterization, and monitor treatment responses, which supports personalized medicine and improves patient outcomes. Additionally, AI-powered systems can identify minor genetic changes and rare biomarkers, such as circulating tumor DNA (ctDNA) and exosomes, which may not be detected by conventional methods. This integration enables more precise diagnostics, real-time monitoring of treatment response, and development of tailored therapeutic strategies. Therefore, the aforementioned factors are expected to positively impact the market growth in upcoming years.
Liquid Biopsy Market Dynamics - (DRO) :
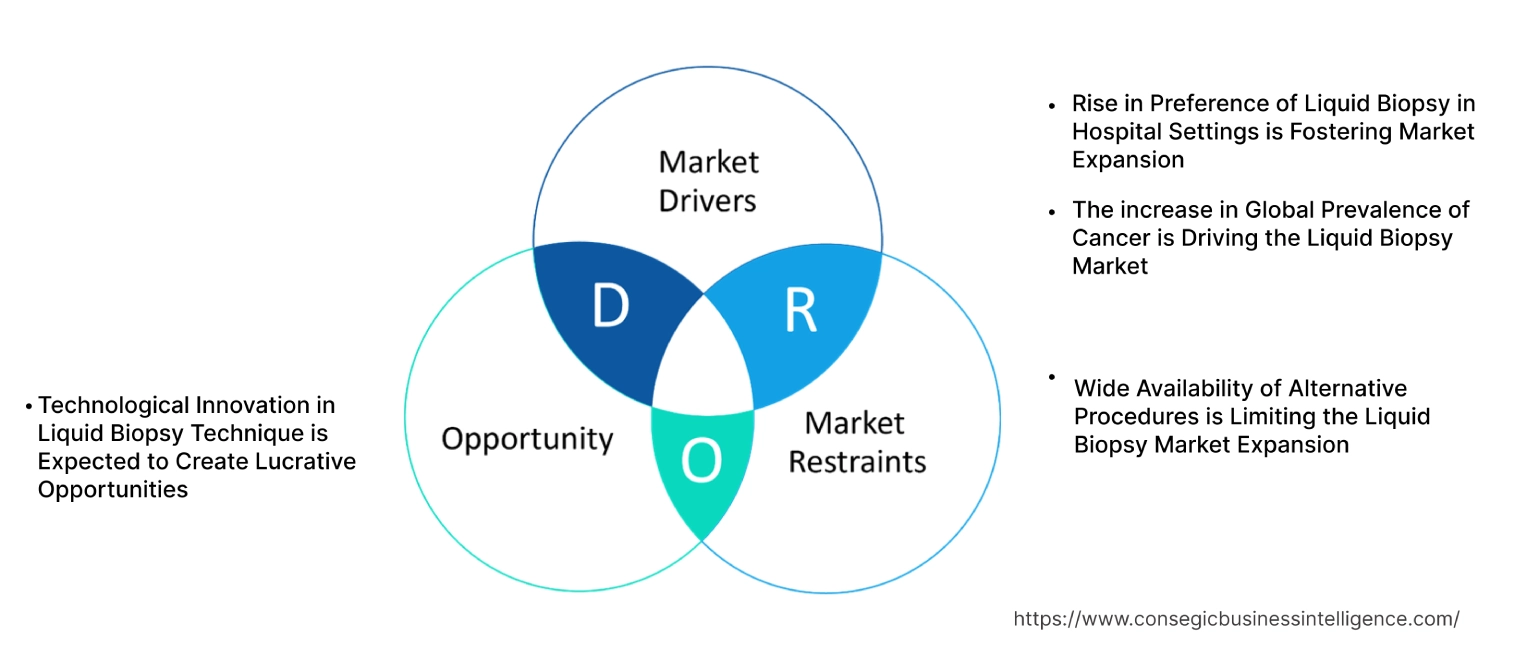
Key Drivers:
The increase in Global Prevalence of Cancer is Driving the Liquid Biopsy Market
Globally, liquid biopsy is being used as a novel diagnostic procedure for cancer detection. It allows minimally invasive molecular characterization of cancer cells. It also helps in monitoring the patient's response to therapy. Strategies include the detection and monitoring of circulating tumor cells, cell-free DNA, and extracellular vesicles, among others. Furthermore, this technique has also been found to be beneficial in the diagnostics of inaccessible cancers, such as brain tumors.
- According to the World Health Organization, in 2022, there were an estimated 20 million new cancer cases and 9.7 million cancer deaths. The estimated number of people who were alive within 5 years following a cancer diagnosis was 53.5 million. About 1 in 5 individuals develop cancer in their lifetime, approximately 1 in 9 men and 1 in 12 women die from the disease.
As there is an increasing preference for the usage of liquid biopsy in cancer patients for detection purposes, the rise in the number of cancer patients in turn leads to a higher adoption rate. Thus, as per the analysis of the market trend, due to the increase in the global prevalence of cancer, the liquid biopsy market share is expanding.
Rise in Preference of Liquid Biopsy in Hospital Settings is Fostering Market Expansion
Globally, in hospitals liquid biopsy is opted as a faster alternative to conventional tissue biopsy. The most significant advantage is its non-invasive nature, requiring only a blood draw, which minimizes patient risk and discomfort. This technique is performed with more frequency, further allowing real-time monitoring of cancer or tumor evolution. It helps in early detection even possibly before the cancer is detectable by other means. Furthermore, it also helps healthcare professionals to understand the treatment response and guides the adjustments if required in therapy.
- In 2023 according to the National Center of Biotechnology Information, liquid biopsy shows a higher success rate compared to tissue biopsy with results approximately 26 days faster than tissue biopsy.
Thus, due to increased efficiency, there is a rise in the trend for liquid biopsy market growth globally.
Key Restraints :
Wide Availability of Alternative Procedures is Limiting the Liquid Biopsy Market Expansion
The presence of alternative procedures, such as tissue biopsy, ultrasound-guided biopsy, endoscopy, vacuum-assisted breast biopsy, and punch biopsy, among others are limiting the market growth of liquid biopsy techniques. Tissue biopsy as an alternative technique offers a superior diagnostic accuracy. Due to the presence of alternatives, there is an increase in competition. Furthermore, the accuracy of the technique depends upon the type and stage of cancer. The technique also lacks the comprehensive cellular architecture details that are obtained from tissue biopsies.
This assay has a tendency to miss critical genetic alterations in early disease states simply because of low concentrations of tumor ctDNA. This results in a delay in the diagnosis and administration of crucial lifesaving therapeutics. Additionally, due to the presence of ctDNA from non-tumor sources, such as clonal hematopoiesis of indeterminate potential (CHIP), complicates the interpretation of results obtained from this technique. Therefore, due to the availability of alternatives, the liquid biopsy market growth is limited.
Future Opportunities :
Technological Innovation in Liquid Biopsy Technique is Expected to Create Lucrative Opportunities
The implementation of artificial intelligence (AI) and machine learning (ML) powered tools for detecting circulating tumor DNA (ctDNA) in blood shows unprecedented sensitivity in predicting the occurrence of cancer. AI and machine learning-directed liquid biopsy have the potential to predict the recurrence of the cancer DNA. Additionally, the detection of ctDNA holds great potential. As detection requires a matched tumor sample, this presents a lucrative opportunity for AI and ML-based liquid biopsy for higher adoption in routine diagnosis.
Diagnostic centers are incorporating AI and machine learning such as MRD-EDGE for detecting cancer. MRD-EDGE is an ultra-sensitive machine learning guided ctDNA analysis platform for detection in low tumor fraction cancer.
- In 2024 according to the National Centre of Biotechnology Information, MRD-EDGE leverages the breadth of plasma WGS to increase liquid biopsy sensitivity. Broadly, MRD-EDGESNV uses advanced machine learning and biologically-informed feature space to enrich the ctDNA signal. Further studies have also shown MRD-EDGE detecting five patients who had a colorectal cancer recurrence, without any false negatives.
Thus, the technological advancement in this technique, due to its increased efficacy and efficiency is expected to drive the liquid biopsy market opportunity in upcoming years.
Liquid Biopsy Market Segmental Analysis :
By Cancer Type:
Based on cancer types the segment is categorized into lung cancer, breast cancer, colorectal cancer, prostate cancer, and others.
Trends in the Cancer Type:
- Increase prevalence of lung cancer globally, liquid biopsy is observing significant growth.
- Liquid biopsy helps prevent the recurrence of breast cancer, by routine monitoring of treatment response.
Lung cancer accounted for the largest market share of the total liquid biopsy market share in 2023.
- In order to tackle the increasing global prevalence of lung cancer, the use of liquid biopsy is increasing among healthcare facilities.
- This technique uses a simple blood draw, rather than excising tissue from a patient since fragments of tumor DNA are shed into the bloodstream.
- In 2023, Illumina and 13 other organizations made liquid biopsies available to 1,260 lung cancer patients consecutively across the United States, under a partnership named QuicDNA.
- Thus, due to the increase in the prevalence of lung cancer globally, the use of this technique for lung cancer diagnosis holds the largest market share.
The breast cancer segment is anticipated to register the fastest CAGR growth during the forecast period.
- Globally, liquid biopsy is rising to have opted for diagnosis and screening, prediction of prognosis, and early relapse detection in localized and locally advanced breast cancer.
- This technique is slowly gaining attention as a non-invasive methodology, for breast cancer diagnosis.
- Liquid biopsy blood test, for detecting the PIK3CA mutation, is the only test approved by the FDA for breast cancer and used in clinical practice.
- In 2023 according to the National Center of Biotechnology Information, liquid biopsy sensitivity to detect breast cancer with stage 1 in health care was found to be <10%, 50% with stage 2, and >80% with stage III or IV breast cancer. Due to the increased rate of detection, there is a rise in the number of oncologists opting for this technique to detect breast cancer.
- Thus, the segmental trend analysis shows that, due to the increased rate of detention the market for this technique among oncologists is growing.
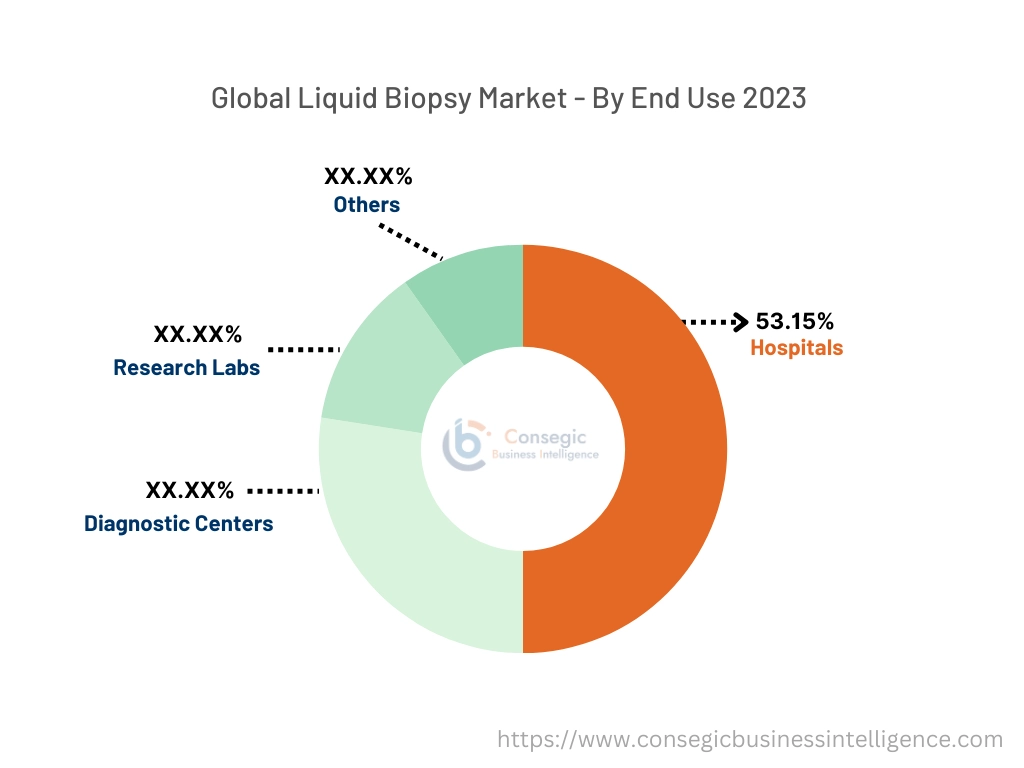
By End-User:
Based on end-use the segment is categorized into hospitals, diagnostic centers, research labs, and others
Trends in the End Use:
- Non-invasive nature of liquid biopsy is rising its demand among patients in hospitals and diagnostic centers.
- Research labs are using liquid biopsy technology to test the newly discovered cancer-associated biomarker.
The hospitals accounted for the largest revenue of the overall market share of 53.15% in the year 2023.
- With the advancement of technology and optimization of methods, liquid biopsy is increasingly applied globally across various hospitals and is becoming a powerful tool for cancer diagnosis and prognosis.
- This technique provides results faster compared to traditional biopsy testing.
- Furthermore, this technique due to its advantage in the detection of circulating tumor DNA, shed by the tumor, in the patient’s blood, is widely used for patients undergoing cancer diagnosis in a hospital.
- According to F. Hoffmann-La Roche Ltd, one of the Swiss multinationals holding the healthcare industry, comprehensive genomic profiling with liquid biopsies involves drawing only two vials of blood, which reduces time spent in hospital, leading to the increased number of individual's preference for liquid biopsy at hospitals.
- Thus, as per the liquid biopsy market analysis, due to the rise in patient preference for this technique, is increasingly being used in hospitals.
The diagnostic center as an end user is anticipated to register the fastest CAGR during the forecast period.
- Globally, an increased number of individuals visit the diagnostic center for routine liquid biopsy, to check their health profile.
- This technical analysis facilitates the detection of clinically relevant tumor mutations from a blood sample when tissue biopsy is not practically possible.
- This technique also helps the diagnostic centers to collect samples from patients who cannot travel to the diagnostic center.
- According to Penn Medicine’s Liquid Biopsy Laboratory, an average of 500 liquid biopsies per month and over 6,000 per year are performed at the diagnostic centers.
- Thus, the use of this technique at the diagnostic center, is poised to be registered as the fastest-growing market.
Regional Analysis:
The regional segment includes North America, Europe, Asia Pacific, the Middle East and Africa, and Latin America.
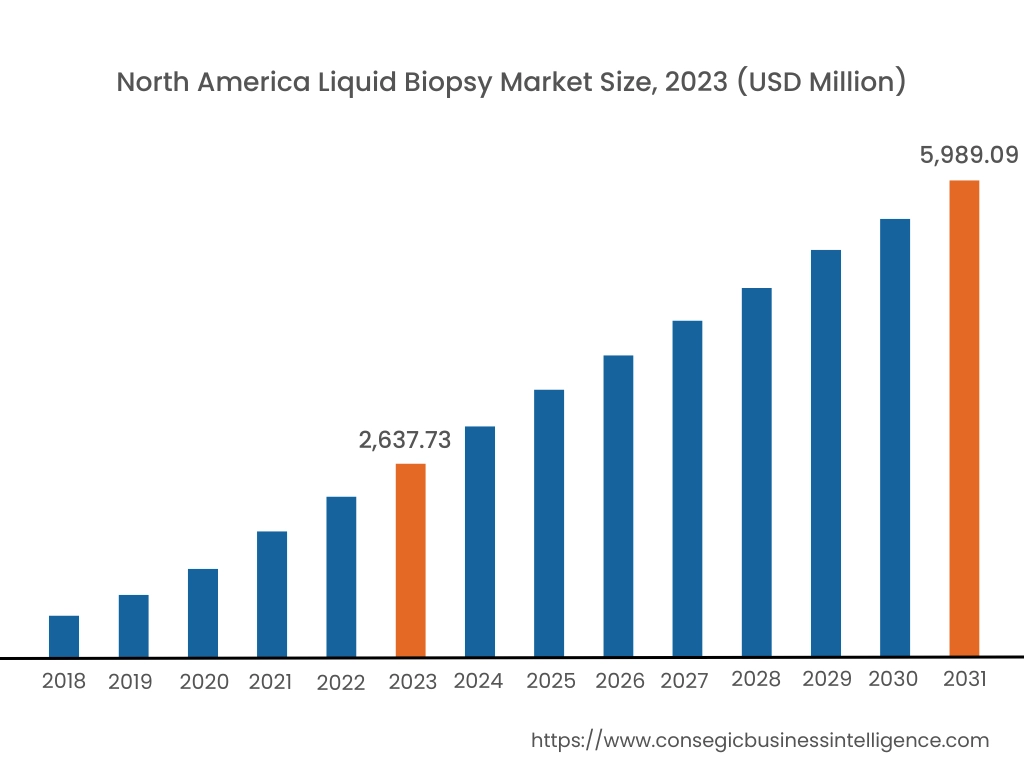
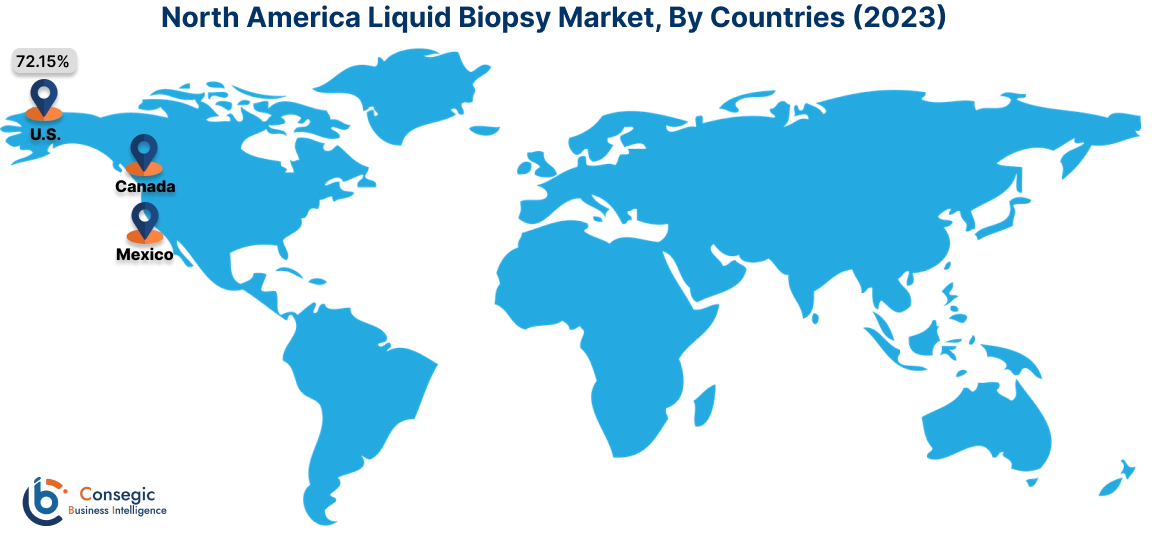
In 2023, North America accounted for the highest market share at 38.15% and was valued at USD 2,637.73 Million, and is expected to reach USD 5,989.09 Million in 2031. In North America, the United States accounted for the highest market share of 72.15% during the base year of 2023. In North America, the test is extensively used to detect cancer, including the detection of tumor cells and cancer cell DNA. FDA-approved tests such as Cell Search® Circulating Tumor Cell (CTC) Test, cobas® EGFR Mutation Test v2, Guardant360® CDx, and FoundationOne® Liquid CDx to detect a few types of advanced cancers, are helping healthcare personnel to predict the prognosis and make quick treatment decisions. Furthermore, as the prevalence of cancer is increasing in the United States, there is increased use of liquid biopsy in the United States.
- In 2024, according to the Centers for Disease Control and Prevention, more than 1.7 million cases of cancer are diagnosed each year in the United States. Liquid biopsy offers a noninvasive procedure in the early detection of cancer. Its use for cancer detection is rising, thereby increasing the survival rate of cancer patients in the United States.
Thus, as per the regional trend analysis, due to the increase in cancer prevalence among individuals, the liquid biopsy market trends are rising in North America.
Asia Pacific is expected to witness the fastest CAGR over the forecast period of 11.4% during 2024-2031. In the Asia Pacific region, there is an increase in the use of liquid biopsy to reduce the recurrence risk of cancer in individuals undergone surgery. In countries such as Japan, China, India, and Australia increased number of individuals are undergoing surgery. Asia Pacific accounts for 45% of all global breast cancer cases and 58% of cervical cancer deaths. Thus, an increasing number of healthcare facilities in the region are opting for the use of this technique to reduce the mortality rate of cancer patients. This technique in the Asia Pacific helps healthcare professionals to closely monitor patients undergoing surgery and prevent the recurrence of cancer. This technique for genetic testing in patients with advanced cancer is already in clinical use in Japan.
- In 2023, according to a study published at the National Cancer Center Japan, the recurrence risk of cancer was determined in 2,240 colorectal cancer patients using liquid biopsy. The results showed that patients with detectable circulating tumor DNA (ctDNA) in post-operative liquid biopsy had a higher risk of cancer recurrence and shorter survival time compared to those without detectable ctDNA.
Thus, as per the regional trend analysis, due to the promising prevention of cancer recurrence in patients, the liquid biopsy market demand is expected to rise exponentially.
In Europe, the European Liquid Biopsy Society aims to ensure that liquid biopsy tests soon become part of clinical routine to benefit cancer patients. This technique is expected to improve patient care in countries such as Germany, France, Finland, the UK, and the Netherlands, among others. This technique is observed as a novel diagnostic strategy for cancer and other diseases. Furthermore, SOPHiA GENETICS one of the leading cloud-native healthcare technology companies and a global leader in data-driven medicine, announced that the company is joining the European Liquid Biopsy Society (ELBS), a prestigious network consisting of partners from academia and industry with the common goal of making LB tests part of the routine standard for patient care. Thus, it is evident that the liquid biopsy market is subjected to substantial growth in the European region.
Brazil represents the largest population in the Latin American region. In Brazil, as well as in other countries from Latin America, oral cancer poses a significant health burden. Oral cavity cancer is known to be associated with lifestyles such as tobacco and alcohol consumption. To treat the high incidence of oral cancer found in lower and middle-income countries, especially in Brazil and Cuba, healthcare facilities are trying to opt for liquid biopsy. This quick and non-invasive approach is found to be useful in the prediction of oral cancer metastasis and monitoring treatment efficacy. Still, the adoption rate of this technique is low in this region. Thus, even though the adoption rate is still low, it is still expected that the market will rise in demand in Latin America.
The use of liquid biopsy is increasing in the Middle East and Africa. In Israel, this new medical technique has witnessed a major surge in adoption and innovation. In Israel, companies such as MetaSight and Senseera are trying to develop screening technology that can detect a wide variety of diseases including cancer from a single blood test. Nevia Bio, a company developing a machine learning-empowered platform for the early detection of women's health, is using LB on vaginal fluid for early detection of diseases is an even more specific utility. This technology is advancing in the Middle East and Africa. Furthermore, startups across the Middle East and Africa are developing LB tests, and the quest for investment is growing even more competitive. Thus, due to the increase in investment for innovation, the LB market is projected for substantial growth in the Middle East and Africa.
Top Key Players & Market Share Insights:
The global liquid biopsy market is highly competitive with major players providing products and services to the national and international markets. Key players are adopting several strategies in research and development (R&D), product innovation, and end-user launches to hold a strong position in the global liquid biopsy market. Key players in the liquid biopsy industry include-
- Guardant Health, Inc. (United States)
- Illumina, Inc. (United States)
- OncoDNA (Belgium)
- Bio-Rad Laboratories, Inc. (United States)
- QIAGEN (Germany)
- Thermo Fisher Scientific Inc. (United States)
- NeoGenomics Laboratories. (United States)
- Hoffmann-La Roche Ltd (Switzerland)
Recent Industry Developments :
Launch:
- In July 2024, Guardant Health, Inc., one of the leading precision oncology Industries, announced the launch of a major upgrade to its market-leading Guardant360 liquid biopsy test. The new enhanced test evaluates biomarkers in 739 genes in total, which is 10 times more cancer biomarkers than the previous version of Guardant360 evaluated. This test can also identify an extensive array of emerging biomarkers to precisely characterize cancer and quantify disease burden at much higher sensitivity. These biomarkers, or gene alterations, detected by the test enable oncologists to identify the targeted treatment strategies, that are most effective for patients with advanced cancer.
- In November 2023, Illumina Inc., one of the global leaders in DNA sequencing and array-based technologies, announced a new generation of its distributed liquid biopsy assay for genomic profiling. The new TruSight™ Oncology 500 ctDNA v2 (TSO 500 ctDNA v2) is a research assay that enables noninvasive comprehensive genomic profiling (CGP) of circulating tumor DNA (ctDNA) from blood when tissue testing is not available or complements tissue-based testing. Key improvements include a faster sample-to-answer turnaround time of less than four days.
Technological Advancements:
- In October 2024, QIAGEN, announced key updates to its sample technologies solutions for non-invasive liquid biopsy applications for use in research and clinical applications such as oncology, prenatal care, and organ transplantation. The introduction of these new kits and the updates to the EZ2 Connect platform underline QIAGEN’s commitment to advancing biopsy technologies.
Partnerships:
- In 2022, C2i Genomics partners with OncoDNA to bring AI-powered liquid biopsy for cancer diagnostics across Europe.
Liquid Biopsy Market Report Insights :
| Report Attributes | Report Details |
| Study Timeline | 2018-2031 |
| Market Size in 2031 | USD 15,495.72 Million |
| CAGR (2024-2031) | 10.7% |
| By Product Type |
|
| By End-User |
|
| By Region |
|
| Key Players |
|
| North America | U.S. Canada Mexico |
| Europe | U.K. Germany France Spain Italy Russia Benelux Rest of Europe |
| APAC | China South Korea Japan India Australia ASEAN Rest of Asia-Pacific |
| Middle East and Africa | GCC Turkey South Africa Rest of MEA |
| LATAM | Brazil Argentina Chile Rest of LATAM |
| Report Coverage |
|
Key Questions Answered in the Report
How big is the Liquid Biopsy Market? +
In 2023, the Liquid Biopsy Market was USD 6,914.09 Million.
Which is the Largest region in the Liquid Biopsy Market? +
North America is the Largest region in the Liquid Biopsy Market.
Which holds the fastest-growing market share for the Liquid Biopsy Market? +
Aisa Pacific is the fastest-growing region in the Liquid Biopsy Market.
What specific segmentation details are covered in the Liquid Biopsy Market? +
Cancer Type and End-Use Industry are the segments that have been covered in the Liquid Biopsy Market.
Who are the major players in the Liquid Biopsy Market? +
Guardant Health, Inc. (United States), Illumina, Inc. (United States), QIAGEN (Germany), Thermo Fisher Scientific Inc. (United States), NeoGenomics Laboratories. (United States), F. Hoffmann-La Roche Ltd (Switzerland), OncoDNA (Belgium), Bio-Rad Laboratories, Inc. (United States).