- Summary
- Table Of Content
- Methodology
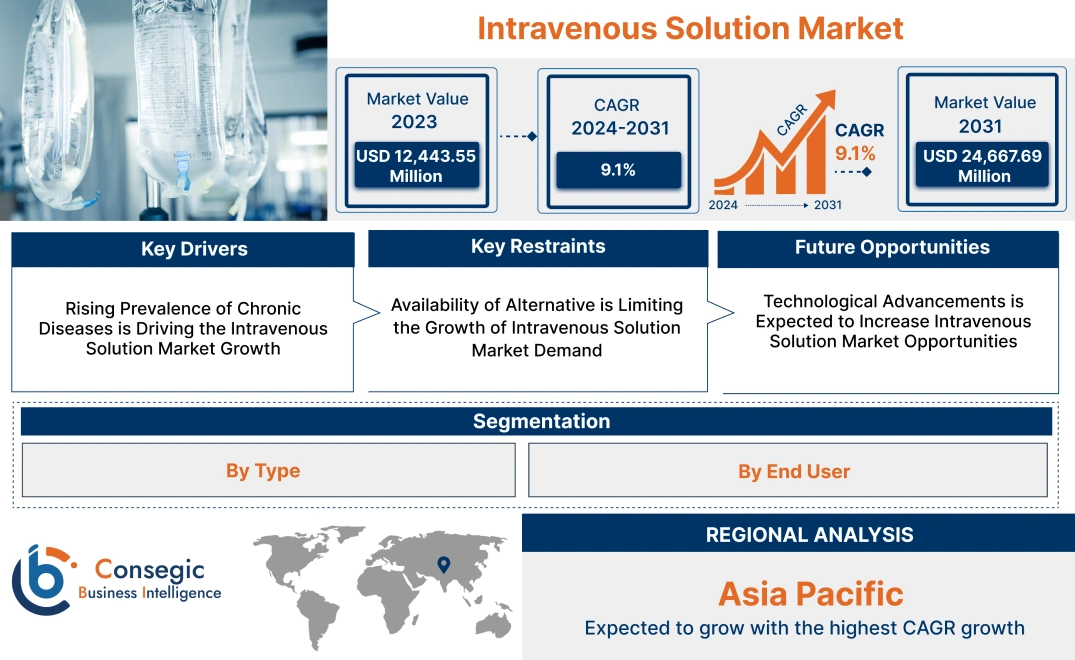
Intravenous Solution Market Size:
Intravenous Solution Market size is estimated to reach over USD 24,667.69 Million by 2031 from a value of USD 12,443.55 Million in 2023, growing at a CAGR of 9.1% from 2024 to 2031.
Intravenous Solution Market Scope & Overview:
Intravenous (IV) solutions are sterile fluids, injected into a person's veins through an IV (intravenous) tube. It is intended for use in patient care. They are used to prevent or treat dehydration, electrolyte imbalances, nutrient deficiency, and others. IV solutions are primarily preferred for individuals especially those who are sick, injured, and dehydrated.
They are broadly classified into two types crystalloid solutions and colloids. Crystalloid solutions are the most common type of IV fluid. They contain small, dissolved molecules that pass easily through semipermeable membranes such as tissues or cells. Some examples of crystalloid solutions are normal saline, Dextrose 5% in Water (D5W), lactated Ringer's, and more.
Colloids in IV solutions are fluids containing large molecules, such as proteins or synthetic substances. These large molecules stay in the bloodstream longer than smaller molecules in crystalloid solutions. Types of colloids are instance albumin, hetastarch fluids, and others. IV solutions are used in hospitals, clinics, and home care amongst others. One of the main advantages of IV solution is to help deliver concentrated doses of medicine without gastrointestinal absorption.
Intravenous Solution Market Dynamics - (DRO) :
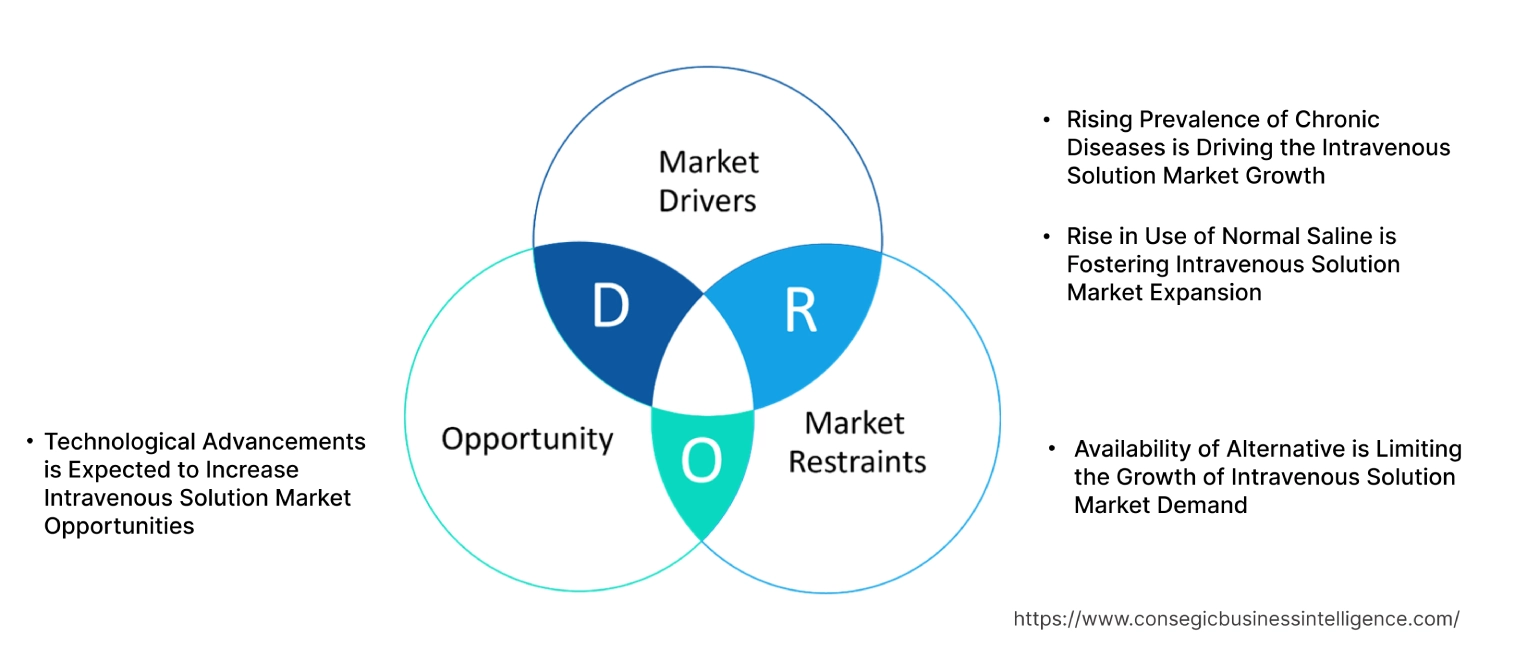
Key Drivers:
Rising Prevalence of Chronic Diseases is Driving the Intravenous Solution Market Growth
Globally, there is an increase in the administration of IV solutions in the treatment of chronic diseases. It is positively impacting patient's health by improving their health condition. It is used for hydration, and nutrition support, and delivers medications that require quick effects upon administration. In chronic diseases, IV solutions help deliver concentrated doses overcoming gastrointestinal absorption barriers which makes it a valuable option for patients with chronic health conditions. Furthermore, globally, there is an increasing prevalence of chronic diseases such as cardiovascular diseases, cancers, chronic respiratory diseases, and diabetes amongst others.
- In 2023 according to the World Health Organization, chronic diseases kill 41 million people each year, equivalent to 74% of all deaths globally. IV solutions increasingly play a crucial role in delivering medication and offering vital support in managing chronic diseases. Thus, the increase in chronic diseases globally shows that there is increased use of IV solutions as it acts as a valuable option in managing chronic illnesses.
Thus, the analysis of market trends depicts that, due to the increased prevalence of chronic disease, the intravenous solution market share is growing.
Rise in Use of Normal Saline is Fostering Intravenous Solution Market Expansion
Normal saline is one of the most common types of crystalloid IV solution. It contains 0.9% sodium chloride. It is commonly used in patient care for hydration purposes. It is also used for diluting injectable medicines. The use of normal saline is increasing due to its similar concentration compared to serum plasma. Additionally, it is majorly used in resuscitation (revival of patients). The manufacturing of normal saline is rising, due to an increase in demand.
- In 2023 according to the National Center of Biotechnology Information, in the United States, 0.9% sodium chloride intravenous (IV) fluid (normal saline) is widely used with more than 200 million liters administered annually.
Thus, as per the analysis of market trends, due to the rising demand for normal saline, the intravenous solution industry size is growing.
Key Restraints :
Availability of Alternative is Limiting the Growth of Intravenous Solution Market Demand
The presence of alternatives such as oral medication, topical treatments, and others is limiting the market growth. Intravenous fluid therapy is a medical technique, in which IV solution is injected into veins to prevent or treat dehydration, whereas in contrast oral medication are preferred alternative by the patients to treat dehydration due to easier administration and less invasive features.
The presence of these alternatives increases competition in the intravenous solution industry. Furthermore, oral medication is cost-effective and less painful while posing lower risks. Owing to this, an increasing number of patients are showing a preference for oral medications. Additionally, oral rehydration therapy is less expensive and does not require hospitalization, which also hinders market growth.
Hence, due to the availability of alternatives, the intravenous solution market growth is restricted.
Future Opportunities :
Technological Advancements is Expected to Increase Intravenous Solution Market Opportunities
Globally, IV solutions have witnessed remarkable technological advancements in recent years. Advancements such as Smart Infusion Pumps, Needle-Free IV Systems, Wearable IV Devices, nanotechnology-derived IV Therapy, and more, are changing the landscape in the field of intravenous treatment, enhancing patient care, and improving healthcare delivery. Needle-free IV system is a pressure-driven and needle-free connector, that delivers medications and IV solutions without the need for needle puncture. Wearable IV devices worn on the body, provide continuous infusion while allowing mobility and freedom for patients. These devices also incorporate smart technologies, such as sensors and real-time data transmission, enabling remote monitoring and enhancing treatment adherence. Additionally, nanotechnology has the potential to transform the field of IV therapy by enhancing drug delivery and targeting specific cells or tissues.
- In 2024 according to Vital IV Therapy, an intravenous (IV) infusion clinic in Melbourne, Smart infusion IV pumps integrated with the IV solution, demonstrate precise and programmable IV fluid delivery, ensuring accurate dosing without compromising patient safety. Beyond the health benefits, smart pumps increase operational efficiency by automating and optimizing the infusion process.
Thus, due to technological advancements, the intravenous solution market opportunities are expected to rise.
Intravenous Solution Market Segmental Analysis :
By Type:
Based on product type the segment is categorized into crystalloids and colloids.
Trends in the Type:
- Rising use of crystalloid and colloid IV solution due to the increase in prevalence of global chronic disease.
- The growing adoption rate of crystalloid IV solutions due to technological advancement, makes the administration convenient for patients.
The crystalloid IV solution accounts for the largest revenue of the total Market share in the year 2023.
- Globally, crystalloid IV solutions are used in the treatment of hypovolemia, hemorrhage, sepsis, and dehydration, these solutions are indispensable for maintaining patient hemodynamic stability.
- It mitigates the risk of nephrotoxicity induced by drugs or toxins.
- There are two sub-types of crystalloid IV solution that are available to clinicians. One is saline which contains 0.9% sodium chloride and the other is balanced crystalloids such as lactated Ringer's.
- Both balanced crystalloids and saline have been opted for clinical use and scientific examination for more than 100 years.
- Studies also show that it is increasingly used in surgeries for maintaining fluid balance and to ensure hydration during or after the procedure.
- In 2023 according to Arizona IV Medics, one of the mobile IV therapy providers serving the Phoenix and Tucson areas states that crystalloid solutions are the most common, largely used IV solution, due to the overwhelming use in hospitals and healthcare settings globally.
- Thus, as per the analysis of segmental trend, globally crystalloid IV solution is dominating the intravenous solution market.
The Colloids IV solution segment is anticipated to register the fastest CAGR during the forecast period.
- Colloids are widely used in the replacement of body fluid as they contain larger molecules, that help retain fluid within the blood vessel and improve blood volume.
- These solutions are of different types such as blood products (whole blood, platelets, and more) or synthetic products (modified gelatins, dextrans, etherified starches, and more).
- They stay in your bloodstream rather than entering your cells. For this reason, globally its use as plasma expanders as a form of fluid resuscitation in cases of severe hypovolemic shock is rising.
- According to an article published at Medscape, one of the global leading online news platforms for physicians and healthcare professionals, globally colloids are increasingly used in niche conditions where the type of colloid is more significant than its colligative properties.
- Thus, as per the analysis of segmental trends, the colloid IV solution market is expected to rise.
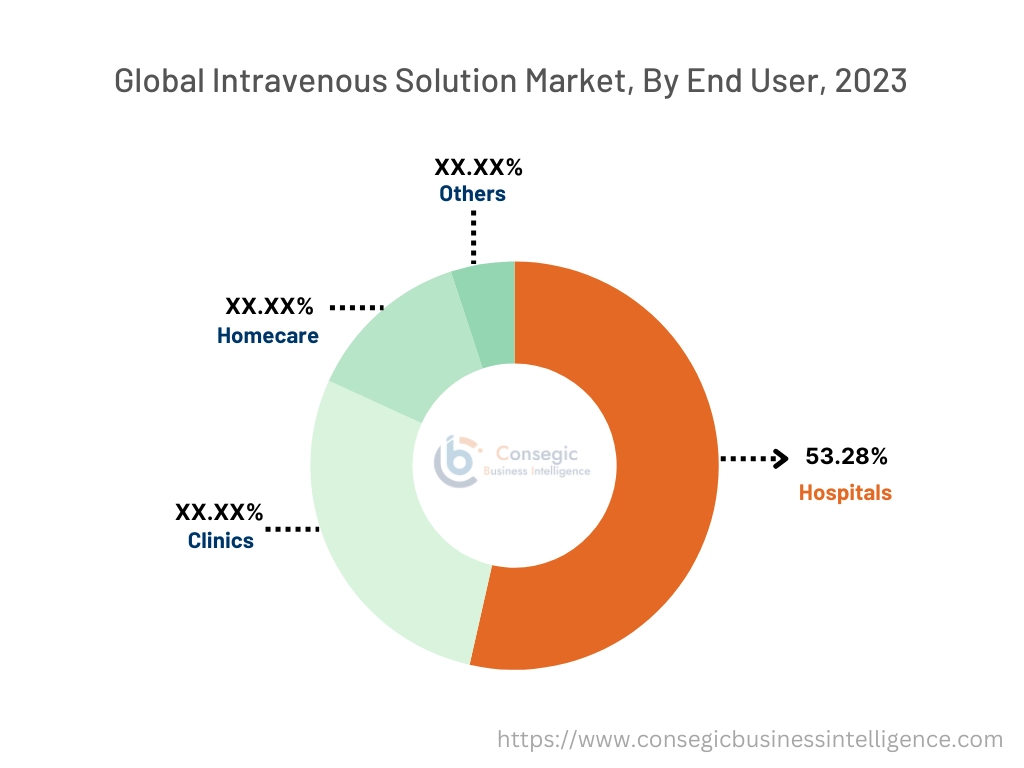
By End User:
Based on end-users the market is segmented into hospitals, clinics, homecare, and others.
Trends in the End User:
- Increase in the need for IV solutions in hospitals to manage conditions such as chronic diseases, electrolyte imbalance, and more.
- Rising patient preference for portable IV solution due to its convenience.
The hospital segment accounted for the largest revenue share of 53.28% in the year 2023.
- The need for IV solutions is increasing in hospitals globally. Its administration is important and remains an essential part of patient care during hospitalization.
- Intravenous (IV) fluid therapy is commonly encountered at hospitals and is increasingly prescribed for patient care.
- In 2023 according to the National Center of Biotechnology Information, globally, approximately 25 million people receive intravenous (IV) therapy by the use of an intravenous cannula at hospitals.
- Thus, segmental analysis of the intravenous solution market trend concludes that the use of IV solutions in hospitals is dominating the intravenous solution market.
The clinics segment is anticipated to register the fastest CAGR during the forecast period.
- Intravenous (IV) therapy is an important part of clinical care.
- It is used to restore fluids, administer blood products or medications, or serve as an alternate route for nutrition when the gastrointestinal tract is not functioning adequately.
- Globally, the number of clinics offering IV therapy is increasing as it offers quick and effective hydration, nutrient replenishment, and more.
- When vitamins are taken orally, the absorption rate is 20-50%. However, it is 90-100% when it is given intravenously.
- Thus, the segmental trend of intravenous solution market analysis depicts that the clinic segment is a fast-growing market segment.
Regional Analysis:
The regions covered are North America, Europe, Asia Pacific, the Middle East and Africa, and Latin America.
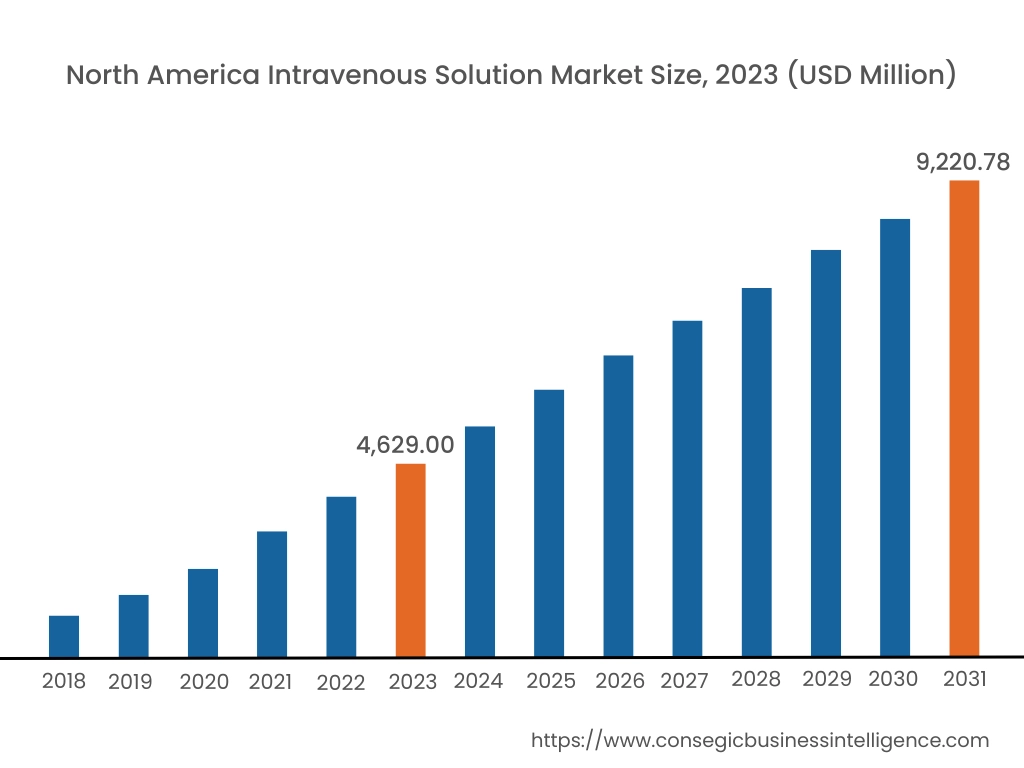
In 2023, North America accounted for the highest intravenous solution market share at 37.20% and was valued at USD 4,629.00 Million, and is expected to reach USD 9,220.78 Million in 2031. In North America, the U.S. accounted for the highest market share of 65.22% during the base year of 2023. An increased number of adult hospital inpatients in North America opt for intravenous (IV) fluid therapy to prevent or treat health problems with their fluid and/or electrolyte status. Intravenous therapy is a treatment that infuses IV solutions, medications, blood, or blood products directly into a vein.
In the United States and Canada, healthcare professionals routinely use IV solutions for acutely ill hospitalized patients. It also helps manage pain and nausea by quickly achieving therapeutic levels. They are more consistently and completely absorbed compared with medications given by other routes such as oral or injection. In the United States, an increased number of healthcare facilities opt for intravenous solutions.
- In 2024 Baxter, an American multinational healthcare company, announced that it would increase the existing allocation percentages of its IV solution in the United States, to cater to the demand from hospitals. The percentages would be from 40% to 60% for direct clients and 10% to 60% for distributors.
Hence, due to the increase in fluid infusion therapy opted by the healthcare facilities, the Intravenous solution market trend is growing in North America.
Asia Pacific is expected to witness the fastest CAGR over the forecast period of 9.8% during 2024-2031. Intravenous solutions are increasingly being used in hospitals across the Asia Pacific region for routine and critical care. They are crucial for fluid replacement, resuscitation, and administering other medications directly into the bloodstream. In countries such as Japan, China, India, Thailand, and Australia companies are investing in additional local IV solution manufacturing capacity, positively influencing intravenous solution market expansion. Additionally, in Thailand increased number of hospital patients are observed to receive peripheral IV fluid administration. Furthermore, there is a rising need for IV solutions in Australia due to market factors such as an increase in hospital admissions for elective surgeries.
- In 2024, Baxter International Inc (Baxter), one of the global leading manufacturers of healthcare products, has recently added additional capabilities to operate at historically high volumes and full capacity to help meet the rising need for intravenous solutions in Australia.
Thus, in the Asia Pacific intravenous solution market analysis, there is increasing production capacity, which is expected to increase the demand in the region.
Europe Commission is providing regulatory approval to therapeutics which involves the use of IV solution. Due to these regulatory approvals, there is a rise in the use of IV solutions in the European region. European Commission (EC) has granted conditional marketing authorization for DURVEQTIX® (fidanacogene elaparvovec), a gene therapy for the treatment of severe and moderately severe hemophilia B (congenital factor IX deficiency) in adult patients without a history of factor IX inhibitors and without detectable antibodies to variant AAV serotype Rh74. This medicinal product is prepared for intravenous solution. Thus, the intravenous solution market demand is poised for a significant rise, due to the regulatory approvals on therapeutic which involve the use of IV solution for dilution.
The requirement for intravenous solutions is increasing in all intensive care unit (ICU) patients in Latin America. They are routinely used in critically ill patients to sustain or replenish intravascular volume and to deliver drug infusions. Studies have found that providing a balanced IV solution to patients in the ICU decreases the rate of mortality. Thus, the growth of the IV solution market in Latin America is fueled by its increased use in ICUs.
There is an increase in the prevalence of sepsis in the Middle East and Africa region. To treat sepsis high concentration antibiotic administration is required. IV solution helps deliver concentrated doses of medicine overcoming the barrier of the gastrointestinal tract. Despite declining age-standardized incidence and mortality, sepsis remains a major cause of health loss worldwide and has an especially high health-related burden in sub-Saharan Africa. Thus, in order to treat sepsis increased number of hospitals in UAE, Israel, Dubai, Africa and more are opting for the use of IV solutions to treat sepsis. It helps in the fast delivery of antibiotics, as well as it helps boost blood pressure and oxygen delivery to tissues. Hence, due to the increase in the perseverance of sepsis, the Middle East and African intravenous solution is expected to witness exponential growth in upcoming years.
Top Key Players & Market Share Insights:
The intravenous solution market is highly competitive with major players providing products and services to the national and international markets. Key players are adopting several strategies in research and development (R&D), product innovation, and end-user launches to hold a strong position in the global intravenous solution market. Key players in the intravenous solution industry include-
- Baxter
- AdvaCare Pharma®
- Puerto Life Sciences Pvt. Ltd.
- Fresenius Kabi AG
- ICU Medical, Inc.
- B. Braun Medical Inc.
- JW Holdings
- Pfizer Inc.
- Otsuka Pharmaceutical Factory, Inc.
Recent Industry Developments :
Business Expansions:
- In 2024, Baxter announced that it is increasing the current U.S. allocation levels of its highest-demand IV fluids for direct customers from 40% to 60%, and for distributors from 10% to 60%. Baxter expects to be at 90% to 100% allocations by the end of the year.
- In October 2024, B. Braun Medical Inc., one of the leading industries of healthcare products, is ramping up production of IV fluids at the manufacturing facilities located in Irvine, CA, and Daytona Beach, FL. In the coming weeks, production levels at those facilities are expected to increase by 20 percent. Additionally, B. Braun is working closely with the Administration for Strategic Preparedness and Response (ASPR) to leverage their resources to move 60 truckloads containing 70,000 cases of IV solutions to a secure facility north of Florida.
Approvals:
- In September 2023, Eisai Co., Ltd. and Biogen Inc. announced that humanized anti-soluble aggregated amyloid-beta (Aβ) monoclonal antibody "LEQEMBI Intravenous Infusion" (200 mg, 500mg, lecanemab) has been approved in Japan as a treatment for slowing progression of mild cognitive impairment (MCI) and mild dementia due to Alzheimer's disease (AD).
- In 2022, Asahi Kasei Pharma today obtained approval to manufacture and sell Cresemba™ Capsule 100 mg and Cresemba™ Intravenous Infusion 200 mg (generic name: isavuconazonium sulfate, development code: AK1820) in Japan for treatment of the fungal infections of aspergillosis, mucormycosis, and cryptococcosis.
Mergers and Acquisitions:
- In 2022, Fresenius Kabi closed the acquisition of Ivenix, Inc. ("Ivenix"), a specialized infusion therapy company. The combination of Ivenix' leading hardware and software products with Fresenius Kabi's offering in intravenous fluids and infusion devices will create a comprehensive and leading setup of premium products. Significant scale and growth synergies are expected by this merger.
Intravenous Solution Market Report Insights :
| Report Attributes | Report Details |
| Study Timeline | 2018-2031 |
| Market Size in 2031 | USD 24,667.69 Million |
| CAGR (2024-2031) | 9.1% |
| By Type |
|
| By End-User |
|
| By Region |
|
| Key Players |
|
| North America | U.S. Canada Mexico |
| Europe | U.K. Germany France Spain Italy Russia Benelux Rest of Europe |
| APAC | China South Korea Japan India Australia ASEAN Rest of Asia-Pacific |
| Middle East and Africa | GCC Turkey South Africa Rest of MEA |
| LATAM | Brazil Argentina Chile Rest of LATAM |
| Report Coverage |
|
Key Questions Answered in the Report
How big is the Intravenous Solution Market? +
Intravenous Solution Market size is estimated to reach over USD 24,667.69 Million by 2031 from a value of USD 12,443.55 Million in 2023, growing at a CAGR of 9.1% from 2024 to 2031.
Which is the fastest-growing region in the Intravenous Solution Market? +
The fastest-growing region in the Intravenous Solution Market is Asia Pacific.
What specific segmentation details are covered in the intravenous solution market report? +
The specific segments that are covered in the intravenous solution market are type and end-user.
Who are the major players in the Intravenous Solution Market? +
Baxter., AdvaCare Pharma, B. Braun Medical Inc., Puerto Life Sciences Pvt. Ltd., Fresenius Kabi AG, ICU Medical, Inc., JW Holdings, Pfizer Inc., Otsuka Pharmaceutical Factory, Inc.