- Summary
- Table Of Content
- Methodology
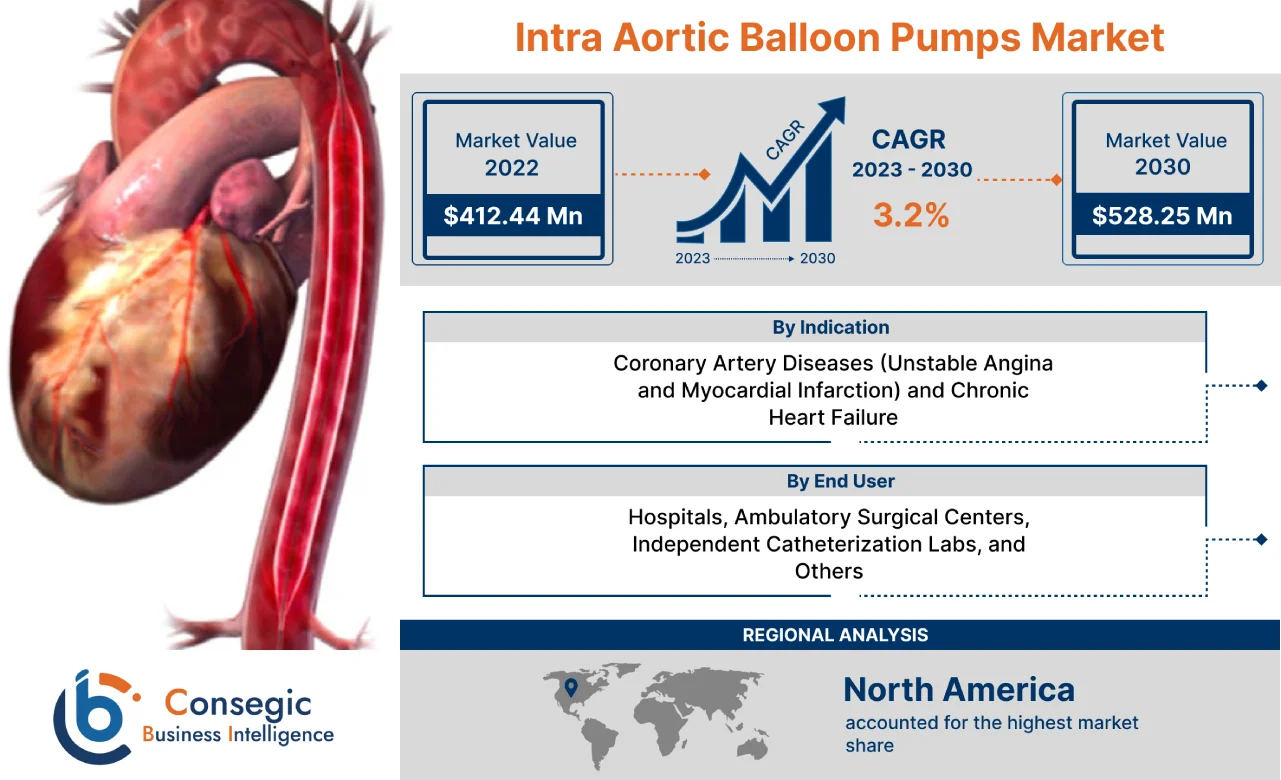
Intra Aortic Balloon Pumps Market Size :
Consegic Business Intelligence analyzes that the intra aortic balloon pumps market size is growing with a healthy CAGR of 3.2% during the forecast period (2023-2030), and the market is projected to be valued at USD 528.25 Million by 2030 from USD 412.44 Million in 2022.
Intra Aortic Balloon Pumps Market Scope & Overview:
The intra-aortic balloon pump is a category of therapeutic device that enables the patient's heart to pump an adequate amount of blood. The intra-aortic balloon pump (IABP) is employed in case of person's heart is incapable of pumping an appropriate amount of blood for the requirements of the body. The intra-aortic balloon pump (IABP) therapy is deployed for the treatment of cardiogenic shock, which is a condition when the heart is unable to pump an adequate amount of blood in order to meet the needs of the body. The primary benefit of an intra-aortic balloon pump (IABP) is it enables blood to flow more efficiently into the coronary arteries.
In addition, an intra-aortic balloon pump (IABP) IABP is frequently used to help patients to recover from surgery related to reopening or bypass of blocked arteries near the heart. Intra aortic balloon pumps are majorly utilized in the treatment of unstable angina, myocardial infarction, and chronic heart failure.
Intra Aortic Balloon Pumps Market Insights :
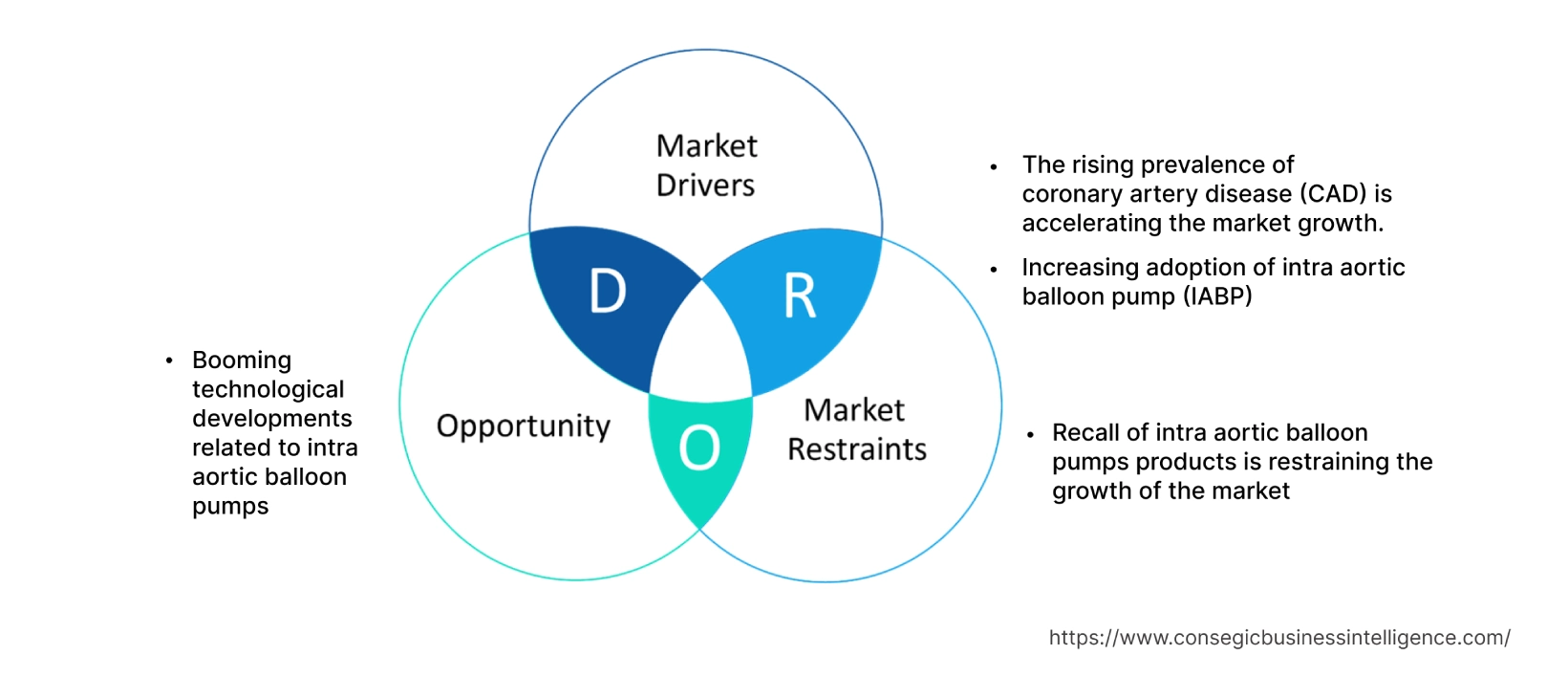
Key Drivers :
The rising prevalence of coronary artery disease (CAD) is accelerating the market growth
The major benefit associated with the employment of an intra aortic balloon pump (IABP) includes it enhances the blood flow to and from the heart. Further, it reduces the amount of energy the heart has to expend. Thus, the intra aortic balloon pump (IABP) is an ideal solution for the treatment of coronary artery disease (CAD). The increasing prevalence of coronary artery disease (CAD) is accelerating the utilization of intra aortic balloon pumps (IABP).
For illustration, according to the recent statistics published by the Centers for Disease Control and Prevention (CDC), in 2021, approximately 1 out of 20 adults in the age group of 20 or more are suffering from coronary artery disease (CAD) in the United States. Also, the expenses associated with heart disease in the United States were approximately USD 239.9 billion per annum. This cost includes the expenses related to medicines, health care services, devices, and others. Hence, the increasing prevalence of coronary artery disease (CAD) is boosting the deployment of intra aortic balloon pumps (IABP) for efficient treatment, which, in turn, is favoring the market growth.
Increasing adoption of intra aortic balloon pump (IABP)
The intra aortic balloon pump (IABP) supports the heart indirectly by minimizing the afterload and increasing the diastolic aortic pressure, thereby boosting the diastolic blood flow. This process leads to efficient perfusion of the peripheral organ along with improvement in coronary blood flow. As a result, intra-aortic balloon pumps (IABP) are frequently deployed in hospitals for the treatment of heart-related diseases.
For instance, various hospitals in India such as Apollo Hospital Chennai Mumbai, Artemis Hospital, Indraprastha Apollo Hospital, New Delhi, Max Healthcare, BLK Super Speciality Hospital, New Delhi, and others are some of the prominent hospitals that are providing intra aortic balloon pump treatment. These players are actively investing to form strategic collaborations with major intra aortic balloon pump players to increase the product offering in hospitals. Hence, the increasing utilization of intra aortic balloon pumps in hospitals to increase systemic blood flow among patients is fostering market growth.
Key Restraints :
Recall of intra aortic balloon pumps products is restraining the growth of the market
The prominent factors such as the concerns associated with fluid leak, distorted packaging, government interventions, non-sterility, and others are leading to the recall of the intra-aortic balloon pump product range. For instance, in December 2022, Datascope, a party of Getinge, recalled Rescue IABPs and Cardiosave Hybrid IABPs, a range of intra-aortic balloon pumps. The prime reason for the recall of the intra aortic balloon pump was because of the leakage in the balloon. The leakage in ballon can impact the blood entering into intra aortic balloon pump.
Moreover, in December 2021, the U.S. Food and Drug Administration (FDA) recalled intra aortic balloon pump product range manufactured by companies, including Getinge, owing to fluid leak concerns. Thus, due to the above factors international agencies such as FDA are recalling various intra aortic balloon pump products. The recall of intra aortic balloon pump products will impact the revenue growth of various companies, thereby posing a major bottleneck for market growth in the upcoming year.
Future Opportunities :
Booming technological developments related to intra aortic balloon pumps
The major players dealing in the intra aortic balloon pump market are increasingly leveraging their technological potential for the development of a new range of intra aortic balloon pumps to increase their product offerings in the market. As a result of this, intra aortic balloon pump manufacturers, having their presence at the global level, are continuously investing in the development of new methods with updated technological advancements to ensure the development of new intra aortic balloon pumps to improve blood flow. Thus, the launch of new intra aortic balloon pumps with upgraded technology is creating a lucrative growth opportunity for the growth in the forecast year.
For instance, in December 2021, Terumo Aortic introduced Aortic Balloon. Hence, the increasing new technological developments of intra aortic balloon pumps by various global companies to enhance the blood flow in the heart is a crucial factor amplifying the growth of the global intra aortic balloon pumps market.
Intra Aortic Balloon Pumps Market Report Insights :
| Report Attributes | Report Details |
| Study Timeline | 2017-2030 |
| Market Size in 2030 | USD 528.25 Million |
| CAGR (2023-2030) | 3.2% |
| By Indication | Coronary Artery Diseases (Unstable Angina and Myocardial Infarction) and Chronic Heart Failure |
| By End User | Hospitals, Ambulatory Surgical Centers, Independent Catheterization Labs, and Others |
| By Region | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
| Key Players | Teleflex Incorporated, Zeon Corporation, Tokai Medical Products, Inc., InterValve Medical Inc., Insightra Medical, Inc., Getinge AB, Terumo Medical Corporation, Avante Medical Solutions Ltd., Abiomed, and MERA |
Intra Aortic Balloon Pumps Market Segmental Analysis :
By Indication :
The indication segment is categorized into coronary artery diseases and chronic heart failure. In 2022, the coronary artery diseases segment accounted for the highest market share in the intra aortic balloon pumps market. The key features related to intra aortic balloon pump (IABP) include superior clinical support, designed and engineered for economy & simplicity, easy-to-connect horizontal ports, and others. These prominent features enable the intra aortic balloon pump to keep pace with the blood flow to the heart. Thus, intra aortic balloon pumps ensure accuracy in time for patients suffering from coronary artery diseases such as unstable angina and myocardial infarction. For instance, according to the World Health Organization (WHO) 2022 Global Stroke Factsheet, the risks associated with the development of stroke (myocardial infarction) have risen by 50% over the last 17 years, and currently 1 out of 4 people at the global level is estimated to have a stroke in their lifetime. Thus, the rising incidents of coronary artery diseases are driving the demand for intra aortic balloon pumps (IABP) to increase diastolic aortic pressure to improve blood flow, this, in turn, is accelerating the market growth.
Furthermore, chronic heart failure is expected to be the fastest-growing segment in the market over the forecast period. This is due to the increasing demand for intra aortic balloon pumps to boost the blood flow in the heart.
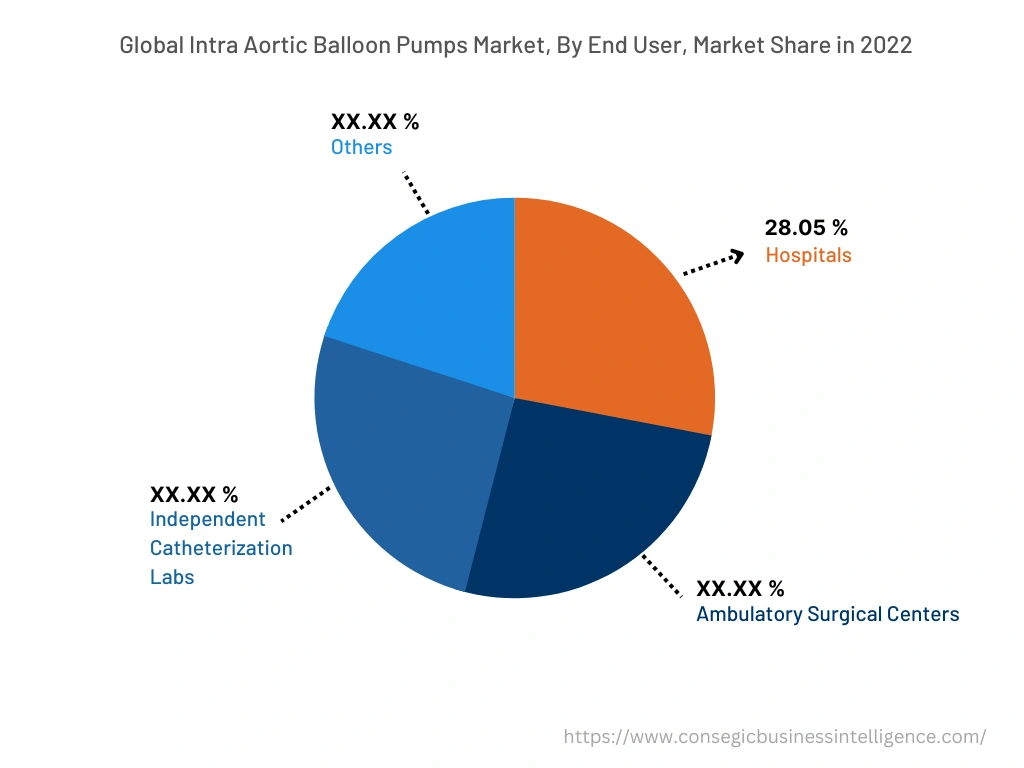
By End-User :
The end user segment is categorized into hospitals, ambulatory surgical centers independent catheterization labs, and others. In 2022, the coronary artery diseases segment accounted for the highest market share of 28.05% in the overall intra aortic balloon pumps market. The intra aortic balloon pump (IABP) is ideal for enhancing cardiac output by approximately 40%. Intra aortic balloon pumps are inserted intraoperatively for heart patients who are incapable of maintaining appropriate blood pressure. The intra aortic balloon pump (IABP) reduces myocardial workload. This leads to efficient blood flow in the heart. As a result, intra aortic balloon pumps (IABP) are utilized across hospitals for high risk percutaneous coronary intervention (PCI). For instance, as of June 2023, various hospitals at the global level, including the American Hospital of Paris, France, Johns Hopkins Hospital, United States, and others are some of the prominent hospitals that are providing intra aortic balloon pump (IABP) treatments. Therefore, the increasing employment of intra aortic balloon pumps in hospitals to ensure efficient treatment of unstable angina, heart attack, and others is fostering the growth of the market..
However, the independent catheterization labs segment is expected to be the fastest-growing segment in the intra aortic balloon pumps market during the forecast period. This is due to the increasing prevalence associated with coronary artery diseases and chronic heart failure at the global level.
By Region :
The regional segment includes North America, Europe, Asia Pacific, Middle East and Africa, and Latin America.
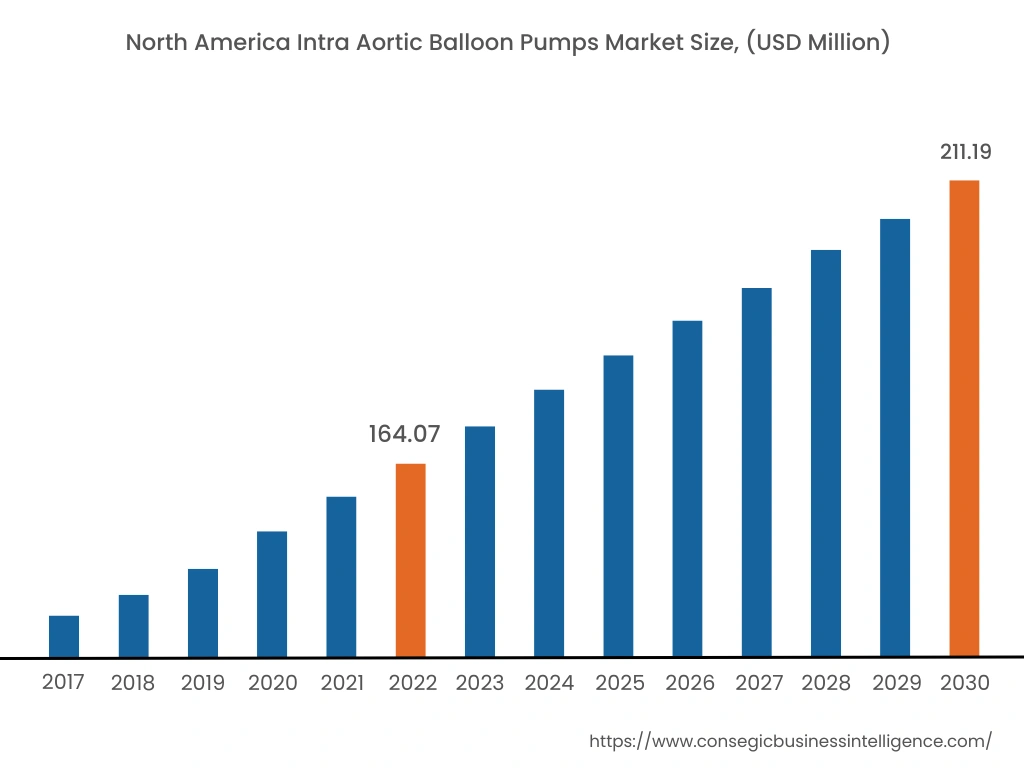
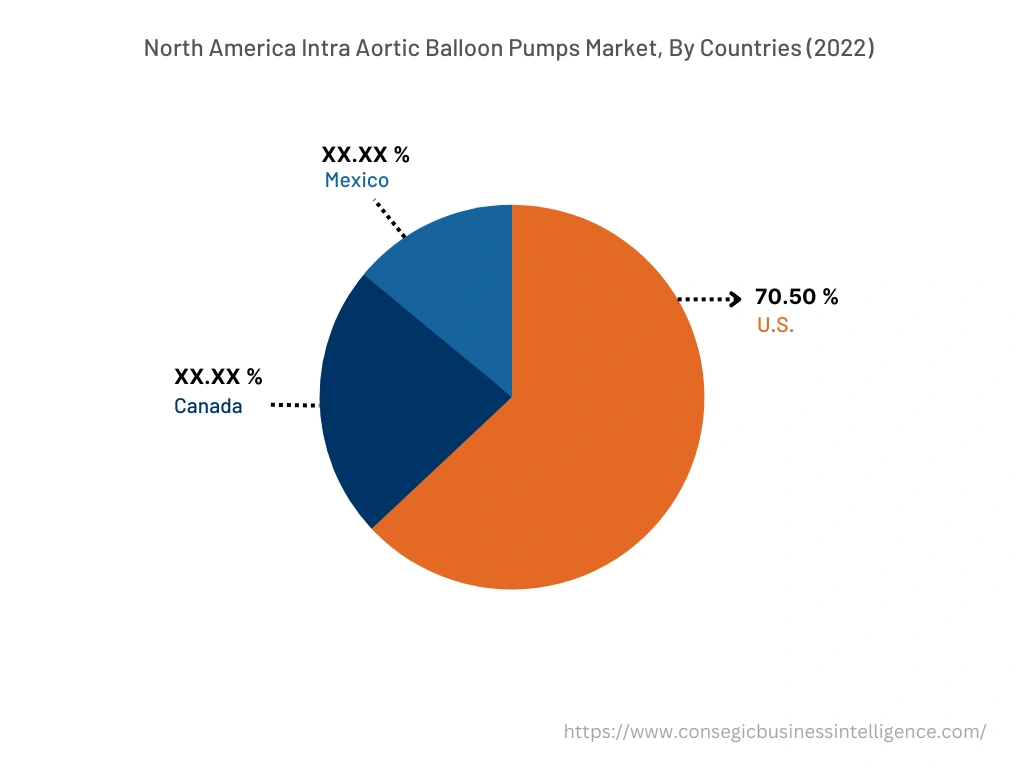
In 2022, North America accounted for the highest market share at 39.78% and was valued at USD 164.07 million, and is expected to reach USD 211.19 million in 2030. In North America, the U.S. accounted for the highest market share of 70.50% during the base year of 2022. The prevalence of heart-related diseases is increasing in the North American region attributed due to the factors such as the increasing aging population, rising diabetes, and others. For instance, according to recent data published by the Centers for Disease Control and Prevention (CDC), approximately 805,000 people in the United States suffer from heart attacks on an annual basis. In addition, about 605,000 cases of heart attack are first-time heart attack while 200,000 occurs in person who have already had a heart attack in the United States. Henceforth, the rise in the prevalence of heart-related diseases in the North American region is expected to boost the demand for intra aortic balloon pumps to enable efficient blood flow, which, in turn, will proliferate the growth of the market.
Furthermore, Asia Pacific is expected to witness significant growth over the forecast period, growing at a CAGR of 3.6% during 2023-20230. This is attributed to the growing demand for intra aortic balloon pumps for the efficient treatment of heart diseases. Therefore, the adoption of intra aortic balloon pumps is increasing in the Asia Pacific for the treatment of unstable angina, myocardial infarction, and others. This factor is proliferating the intra aortic balloon pumps market growth in Asia Pacific.
Top Key Players & Market Share Insights:
The intra aortic balloon pumps market is highly competitive, with several large players and numerous small and medium-sized enterprises. These companies have strong research and development capabilities and a strong presence in the market through their extensive product portfolios and distribution networks. The market is characterized by intense competition, with companies focusing on expanding their product offerings and increasing their market share through mergers, acquisitions, and partnerships. The key players in the market include-
- Teleflex Incorporated
- Zeon Corporation
- Avante Medical Solutions Ltd.
- Abiomed
- MERA
- Tokai Medical Products, Inc.
- InterValve Medical Inc.
- Insightra Medical, Inc.
- Getinge AB
- Terumo Medical Corporation
Recent Industry Developments :
- In December 2022, Johnson & Johnson, headquartered in the United States acquired Abiomed, based in Danvers, U.S. Abiomed is a United States-based manufacturer of intra aortic balloon pumps. The primary focus of Johnson & Johnson with this acquisition was to increase the market position in the global intra aortic balloon pumps market.
- In December 2022, Johnson & Johnson, headquartered in the United States acquired Abiomed, based in Danvers, U.S. Abiomed is a United States-based manufacturer of intra aortic balloon pumps. The primary focus of Johnson & Johnson with this acquisition was to increase the market position in the global intra aortic balloon pumps market.
Key Questions Answered in the Report
What was the market size of the intra aortic balloon pumps industry in 2022? +
In 2022, the market size of intra aortic balloon pumps was USD 412.44 million
What will be the potential market valuation for the intra aortic balloon pumps industry by 2030? +
In 2030, the market size of intra aortic balloon pumps will be expected to reach USD 528.25 million.
What are the key factors driving the growth of the intra aortic balloon Pumps market? +
Increasing adoption of intra aortic balloon pump (IABP) in hospitals.
What is the dominating segment in the intra aortic balloon pumps market by End User? +
In 2022, the hospital segment accounted for the highest market share of 28.05% in the overall intra aortic balloon pumps market.
Based on current market trends and future predictions, which geographical region will have the fastest impact on the intra aortic balloon pumps market's growth in the coming years? +
Asia Pacific is expected to be the fastest-growing region in the market during the forecast period.