- Summary
- Table Of Content
- Methodology
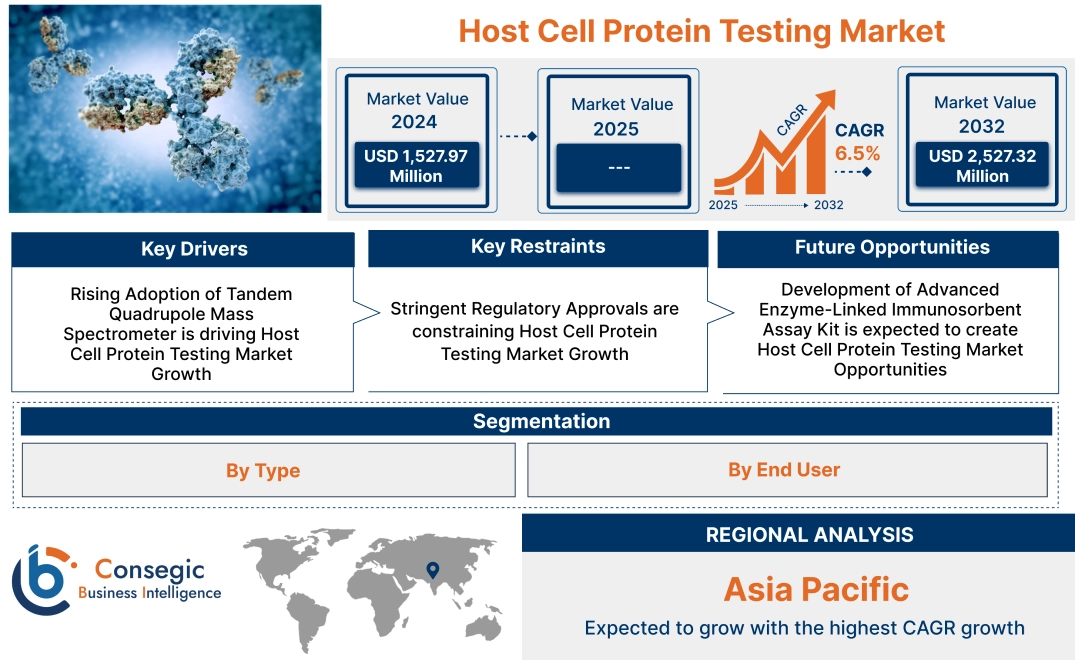
Host Cell Protein Testing Market Size:
Host cell protein testing market size is estimated to reach over USD 2,527.32 Million by 2032 from a value of USD 1,527.97 Million in 2024, growing at a CAGR of 6.5% from 2025 to 2032.
Host Cell Protein Testing Market Scope & Overview:
Host cell protein (HCP) testing is a quality control process used in the biopharmaceutical industry to detect and quantify residual proteins from host cells used during the production of biologics. It ensures that impurities are within acceptable limits to meet regulatory standards and avoid adverse effects in the patients. There are different types of HCP testing such as polymerase chain reaction (PCR)-based assays, enzyme-linked immunosorbent assay (ELISA)-based assays, mass spectrometry-based assays, and others. The mass spectrometry-based assays are further divided into liquid chromatography–mass spectrometry (LC–MS), tandem mass spectrometry (MS/MS), and others. The host cell protein testing is widely used by biopharmaceutical companies, contract research organizations (CRO), and others. Advancements in mass spectrometry-based assays with enhanced precision and sensitivity are further driving the market demand.
Key Drivers:
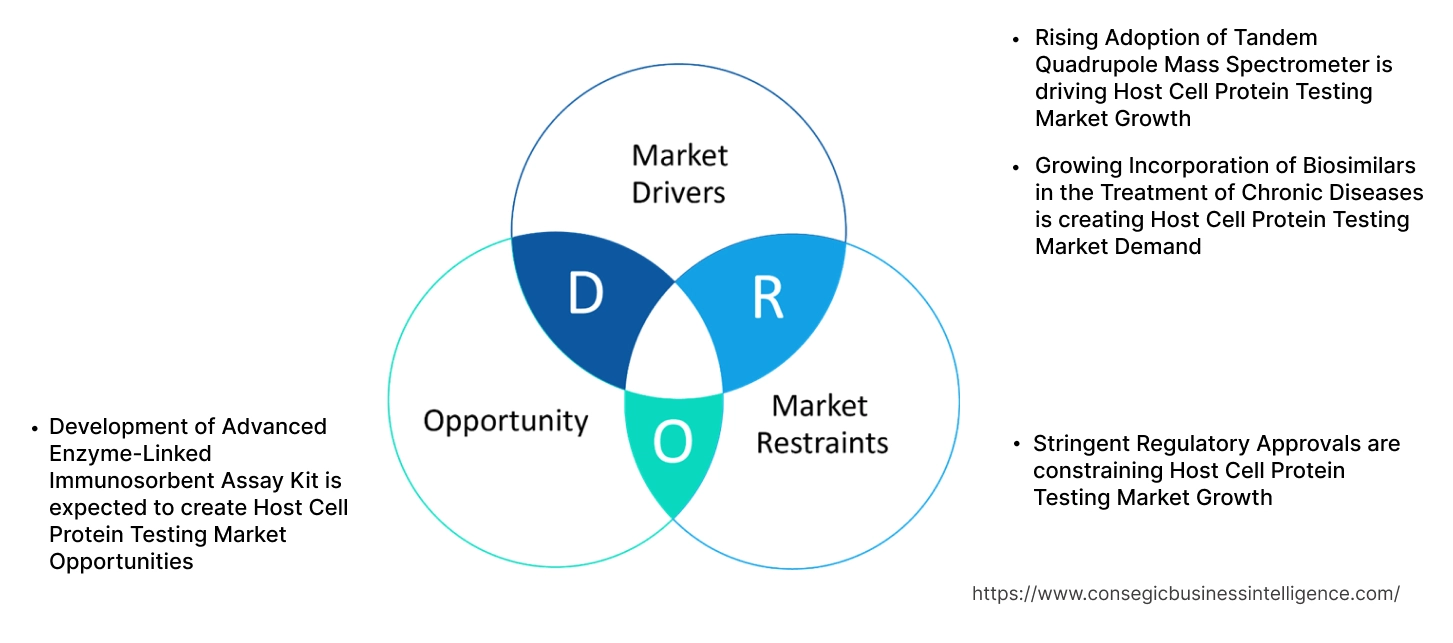
Rising Adoption of Tandem Quadrupole Mass Spectrometer is driving Host Cell Protein Testing Market Growth
Tandem quadrupole mass spectrometer (TQ-MS) is an analytical instrument that uses three quadrupole stages in sequence to identify and quantify molecules based on their mass-to-charge ratio. In host cell protein testing, it is essential to enable precise detection and quantification of residual host protein cells in biologics.
- In 2023, Waters launched Xevo TQ Absolute in vitro diagnostics tandem quadrupole mass spectrometer. It enables high sensitivity which enhances the identification of low abundance host cell proteins in HCP testing. This tandem quadrupole mass spectrometer incorporates sustainability in the market which uses 50% less nitrogen gas and electricity than other TQ-MS systems.
Thus, rising adoption of tandem quadrupole mass spectrometer is leading to host cell protein testing market demand by enhancing sensitivity and identification of low abundance host cell proteins.
Growing Incorporation of Biosimilars in the Treatment of Chronic Diseases is creating Host Cell Protein Testing Market Demand
Biosimilars are biological medical products that are highly similar to an already approved biological medicine. They provide affordable treatment options for chronic diseases such as cancer, diabetes, and rheumatoid arthritis, reducing healthcare costs. Host cell protein testing is essential in biosimilar production by detecting and quantifying residual proteins form host cells.
- In 2022, according to Amgen 2022 BIOSIMILAR TRENDS REPORT, there are 38 biosimilars approved by United States Food & Drug Administration representing 15% increase as compared to the year 2021. Host cell protein testing is essential in biosimilars approval as it identifies & quantifies residual host cell proteins which ensures the safety and efficacy of biosimilars.
Hence, growing incorporation of biosimilars in the chronic diseases treatment is leading to host cell protein testing market expansion by ensuring safety and efficacy in the development and production of biosimilars.
Key Restraints:
Stringent Regulatory Approvals are constraining Host Cell Protein Testing Market Growth
Regulatory approvals are a significant restrain in the market demand due to stringent requirements for the approval of biologics. Some countries have unique requirements for host cell protein testing protocols including acceptable thresholds for residual proteins, validations processes and reporting standards. These validations delay the approval of biologics and biosimilar, as companies must adopt their testing method to meet region-specific criteria. Regulatory agencies like United States Food and Drug Administration, European Medicines Agency, and others enforce stringent quality standards to ensure the safety and efficacy of biologics. Additionally, changing regulations requires huge investment in compliance sources, adding to operational cost.
Thus, regulatory approvals are constraining host cell protein testing market expansion by increasing the time and operational cost for the HCP testing providers.
Future Opportunities :
Development of Advanced Enzyme-Linked Immunosorbent Assay Kit is expected to create Host Cell Protein Testing Market Opportunities
ELISA kit is a laboratory tool used to detect and quantify substances such as proteins, peptides, antibodies and hormones in a liquid sample. It is essential in host cell protein testing to detect and quantify host cell proteins in the biologics manufacturing. By enabling accurate host cell protein detection, ELISA kit facilitates the production of safer and more effective biologics.
- In 2024, ArcticZymes Technologies launched SAN HQ 2.0 ELISA Kit. It is highly sensitive, with a quantification range of 0.2 – 12.8 ng/ml which offers excellent precision in host cell protein testing.
Thus, development of advanced ELISA kit is expected to create host cell protein testing market opportunities by enhancing precision and sensitivity in the testing.
Host Cell Protein Testing Market Segmental Analysis :
By Type:
By type, the market is divided into PCR-based assays, ELISA-based assays, mass spectrometry-based assays, and others. The mass spectrometry-based assays are further divided into liquid chromatography–mass spectrometry, tandem mass spectrometry, and others.
Trends in Type:
- According to host cell protein testing market trends, ELISA-based assays are widely used due to their high accuracy and detection of wide range of contaminants.
- Adoption of mass spectrometry-based assays is growing due to its effectivity in identifying low-abundance host cell protein as per market trends.
The ELISA-based assays accounted for the largest market share in the year 2024.
- The ELISA-based assay is a biochemical technique used to detect and quantify specific proteins or antigens by using colorimetric and fluorescent readout system.
- The ELISA-based assay is the most widely used host cell protein testing due to its high sensitivity, specificity, and accuracy.
- It relies on polyclonal antibodies raised against host cell proteins which enables the simultaneous detection of wide range of contaminants.
- Further, companies are investing in the development of ELISA-based assay kit for reliable quantification during the production and purification process of biologics.
- In 2023, Charles River Laboratories launched ELISA-based assay kit for the detection and quantitation of residual host cell proteins in Chinese hamster ovary based bio therapeutics. It achieves a sensitivity rate of 0.1 ng/mL and specificity, rate of 90 percent antibody coverage in host cell protein testing.
- Thus, ELISA-based assay is dominating in the market due to high accuracy and demand from biopharmaceutical sector.
The mass spectrometry-based assays is expected to grow at the fastest CAGR over the forecast period.
- Mass spectrometry-based assays is an analytical method that uses mass spectroscopy to identify and quantify molecules based on their mass-to-charge ratio, providing high sensitivity and specificity in detecting host cell proteins.
- The adoption of mass spectrometry-based assays is growing in the host cell protein testing due to its effectivity in identifying low-abundance host cell protein, even in complex biological samples.
- The adoption of mass spectrometry-based assays is driven by the increasing complexity of biologics and biosimilar.
- Further, advancements in instrumentation such as tandem mass spectrometry enhance the detection accuracy and speed of mass spectrometry-based assays.
- Therefore, mass spectrometry-based assays are expanding in the market due to high speed and detection accuracy as per market trends.
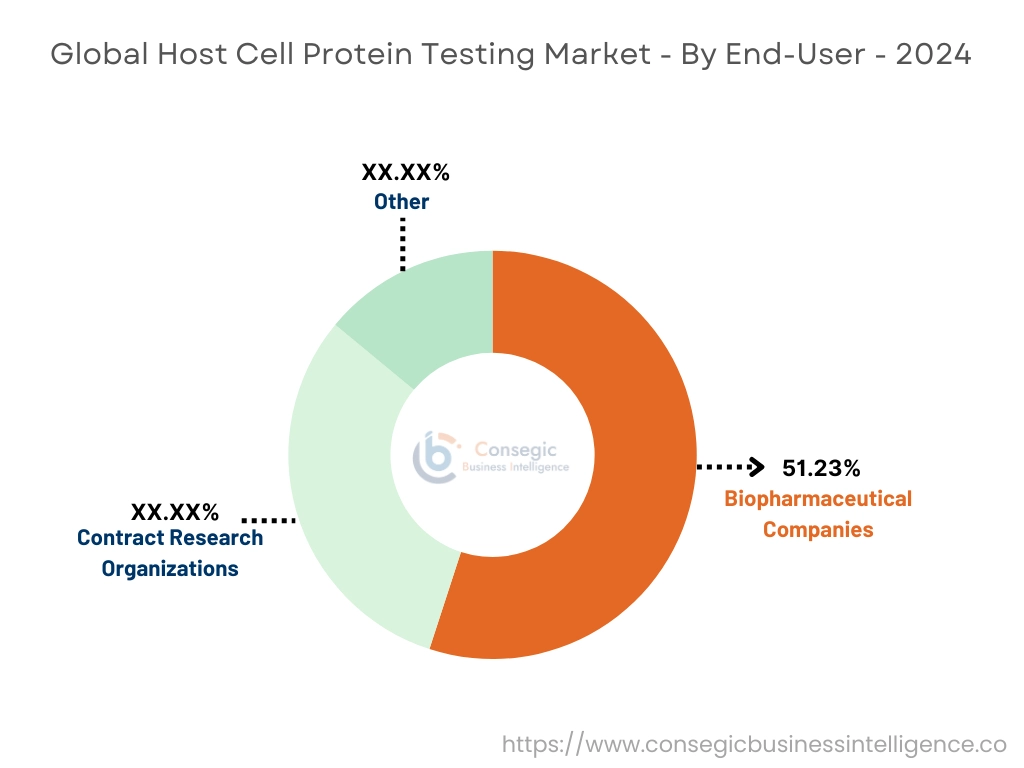
By End User:
By end-user, the market is divided into contract research organizations, biopharmaceutical companies, and others.
Trends in End-User:
- As per host cell protein testing market trends, biopharmaceutical companies widely used host cell protein testing in the development of therapy drugs and vaccines.
- Contract research organizations are rapidly adopting host cell protein testing for quality control processes in the biologics manufacturing as per market trends.
The biopharmaceutical companies accounted for the largest market share of 51.23% in the year 2024.
- Biopharmaceutical companies are organizations that developed therapy drugs and vaccines using biological sources such as living cells, proteins, and nucleic acids.
- Host cell protein testing is widely used by these companies to detect and remove residual host cell proteins that will impact drug safety and stability.
- It also ensures the safety and efficacy of biologics such as monoclonal antibodies, vaccines, and biosimilar.
- Biopharmaceutical companies are incorporating advanced LC-MS systems in host cell protein testing to maintain product quality and comply with regulatory guidelines.
- For instance, in 2024, Agilent Technologies launched Agilent 6495 Triple Quadrupole LC-MS System for host cell protein testing. It features sub-millisecond dwell times and intelligent reflex that provides fast, sensitive, high-quality sample measurements.
- Thus, biopharmaceutical companies are widely incorporating host cell protein testing for drug safety and stability.
The contract research organization is expected to grow at the fastest CAGR over the forecast period.
- A contract research organization is a company that provides support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis.
- Contract research organizations are increasingly adopting host cell protein testing for biologics manufacturing companies that outsource critical quality control processes.
- The growing complexity of host cell protein testing coupled with the need for compliance with stringent regulatory standards are encouraging companies to rely on contract research organization for their expertise and advanced testing capabilities.
- Additionally, contract research organization helps to reduce operational costs and time in the production of biologics further driving the market.
- Thus, contract research organizations are widely adopting host cell protein testing in the production of biologics.
Regional Analysis:
The regional segment includes North America, Europe, Asia Pacific, the Middle East and Africa, and Latin America.
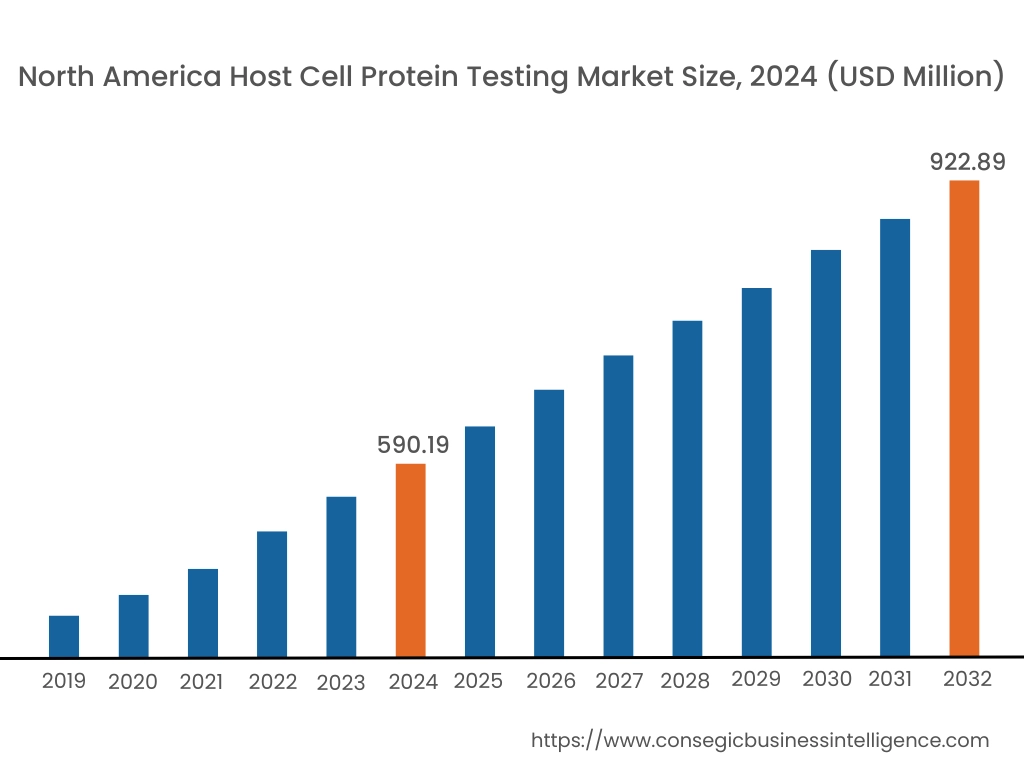
In 2024, North America accounted for the highest market share at 38.63% and was valued at USD 590.19 Million and is expected to reach USD 922.89 Million in 2032. In North America, United States accounted for the highest market share of 72.47% during the base year of 2024.
The host cell protein testing market share of North America is significant due to strong presence of pharmaceutical companies, regulatory bodies, and advanced research facilities. The contract research organizations in the region are investing in the development of advanced mass spectrometers, further boosting the market.
- In 2024, Thermo Fisher Scientific launched Stellar mass spectrometer at American Society for Mass Spectrometry conference in California. This mass spectrometer detects, identifies, and quantifies individual host cell proteins in host cell protein testing.
Thus, North America is leading in the market due to growing investment in research facilities and strong presence of pharmaceutical companies as per analysis.

Asia-pacific is expected to witness the fastest CAGR of 7.6% over the forecast period during 2025-2032.
As per host cell protein testing market analysis, the Asia-pacific region is experiencing rapid growth in the market fueled by rising investments in biotechnology and expanding biopharmaceutical industry. Countries like China, India, and Japan are experiencing rapid growth in biologics and biosimilar production driving demand for host cell protein testing. The regulatory agencies in the region are widely adopting advanced host cell protein testing methods like mass spectrometry, further driving the market. Hence, the host cell protein testing market share of Asia-Pacific is expanding due to expanding biotechnology sector and increasing biosimilar production.
According to host cell protein testing market analysis, Europe region is experiencing considerable growth in the market driven by presence of stringent regulatory bodies and strong biopharmaceutical sector. The regulatory agencies like European Medicines Agency are incorporating host cell protein testing to ensure the safety, quality, and efficacy of biosimilar, biologics, and vaccines. Additionally, the growing focus on personalized medicine and investments in biosimilar development is further driving the market in the region.
The Middle East and Africa region is experiencing gradual growth in the market, driven by expanding biopharmaceutical manufacturing and increasing investments in the healthcare infrastructure. The countries like South Africa, UAE and Saudi Arabia are investing in host cell protein testing to ensure regulatory compliance and product safety. Additionally, the outsourcing of host cell protein testing to contract research organization is growing, further leading to host cell protein testing market proliferation.
As per market analysis, the host cell protein testing market in Latin America is driven by increasing investment in research and growing biosimilar production. Countries like Brazil, Mexico, and Argentina are leading in the market due to their robust pharmaceutical sectors and research facilities. Additionally, the rise of contract research organizations in the region is promoting the outsourcing of host cell protein testing, further contributing to the market growth.
Top Key Players and Market Share Insights:
The host cell protein testing industry is highly competitive with major players providing services to the national and international markets. Key players are adopting several strategies in research and development (R&D) and product innovation to hold a strong position in the global host cell protein testing market. Key players in the host cell protein testing industry include-
- Thermo Fisher Scientific (United States)
- Bio-Rad Laboratories, Inc. (United States)
- Cisbio Bioassays (France)
- Cygnus Technologies LLC (United States)
- Charles River Laboratories (United States)
- Agilent Technologies, Inc. (United States)
- Abcam plc. (United Kingdom)
- Cytiva (United States)
- Enzo Life Sciences Inc. (United States)
- Biogenes GmbH (Germany)
Recent Industry Developments :
Product Launches:
- In 2024, Krishgen Biosystems launched KRIBIOLISA™ High Five (H5) host cell protein ELISA kit. This kit is designed with a direct sandwich assay format to quantitatively measure host cell protein contamination in host cell protein testing.
- In 2024, Gold Standard Diagnostics launched new rapid ELISA test kit for total aflatoxins detection. It enhances the sensitivity, specificity, and efficiency of ELISA-based assays in host cell protein testing.
Mergers & Acquisitions:
- In 2021, Thermo Fisher Scientific acquired PPD, Inc. for USD 17.4 billion. This acquisition allows Thermo Fisher Scientific to expand advanced host cell protein testing to meet growing demand.
Host Cell Protein Testing Market Report Insights :
| Report Attributes | Report Details |
| Study Timeline | 2019-2032 |
| Market Size in 2032 | USD 2,527.32 Million |
| CAGR (2025-2032) | 6.5% |
| By Type |
|
| By End-User |
|
| By Region |
|
| Key Players |
|
| North America | U.S. Canada Mexico |
| Europe | U.K. Germany France Spain Italy Russia Benelux Rest of Europe |
| APAC | China South Korea Japan India Australia ASEAN Rest of Asia-Pacific |
| Middle East and Africa | GCC Turkey South Africa Rest of MEA |
| LATAM | Brazil Argentina Chile Rest of LATAM |
| Report Coverage |
|
Key Questions Answered in the Report
How big is the host cell protein testing market? +
In 2024, the host cell protein testing market is USD 1,527.97 million.
Which is the fastest-growing region in the host cell protein testing market? +
Asia-Pacific is the fastest-growing region in the host cell protein testing market.
What specific segmentation details are covered in the host cell protein testing market? +
Type and end-user are covered in the host cell protein testing market.
Who are the major players in the host cell protein testing market? +
Thermo Fisher Scientific (United States), Bio-Rad Laboratories, Inc. (United States), and Agilent Technologies, Inc. (United States) are some of the major players in the market.