- Summary
- Table Of Content
- Methodology
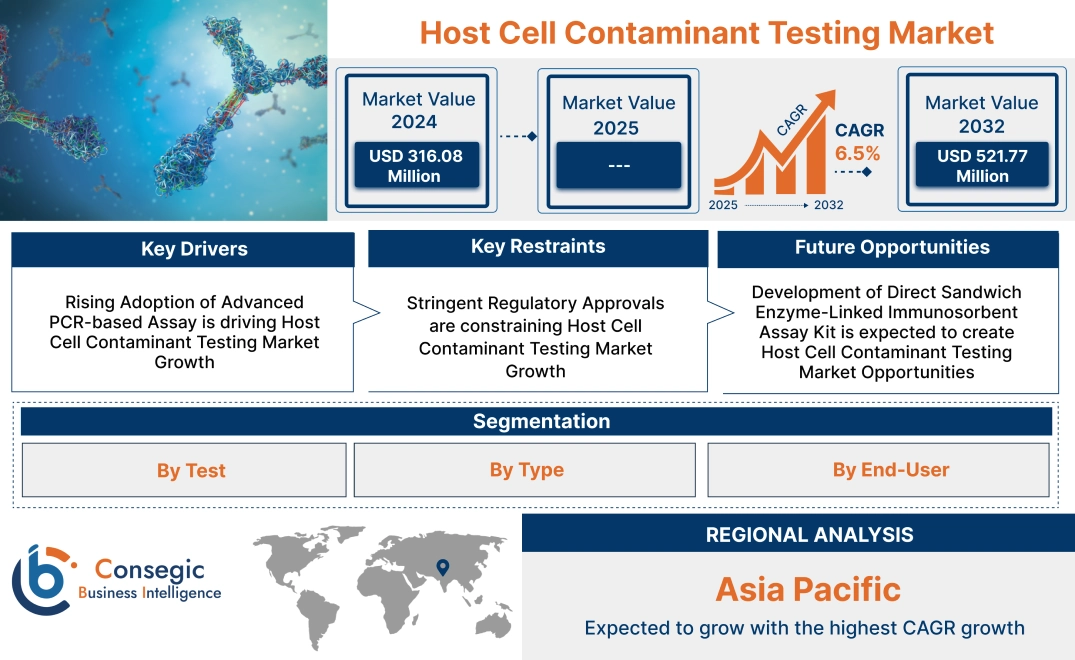
Host Cell Contaminant Testing Market Size:
Host cell contaminant testing market size is estimated to reach over USD 521.77 Million by 2032 from a value of USD 316.08 Million in 2024, growing at a CAGR of 6.5% from 2025 to 2032.
Host Cell Contaminant Testing Market Scope & Overview:
Host cell contaminant testing refers to the process of detecting and quantifying contaminants such as proteins, deoxyribonucleic acid (DNA), endotoxins in host cells during the production of biologics. It ensures that impurities are within acceptable limits to meet regulatory standards and avoid adverse effects in the patients. There are different types of tests in host cell contaminant testing such as enzyme-linked immunosorbent assay (ELISA)-based assay, polymerase chain reaction (PCR)-based assay and others. The host cell contaminant testing is widely used by biopharmaceutical companies, contract research organizations (CRO), contract manufacturing organizations and others. Advancement in polymerase chain reaction- based assays such as digital PCR with enhanced precision and sensitivity is further driving the market demand.
Key Drivers:
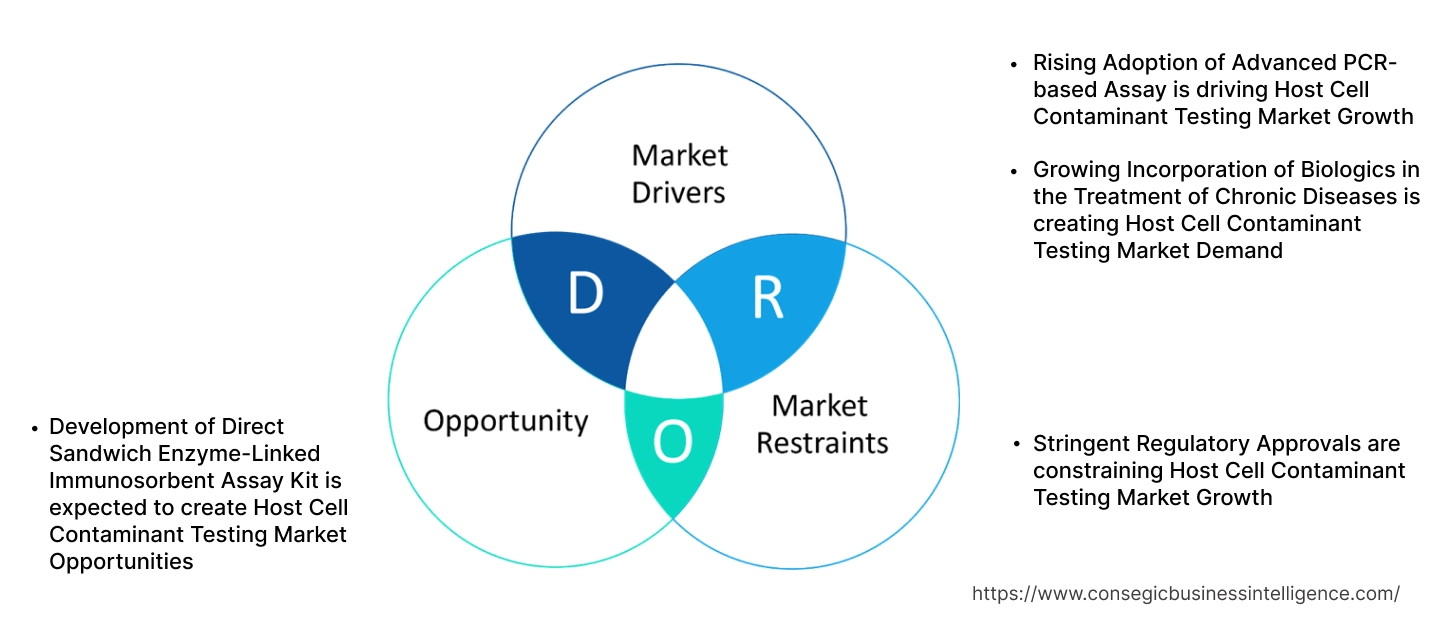
Rising Adoption of Advanced PCR-based Assay is driving Host Cell Contaminant Testing Market Growth
A PCR-based assay is a molecular technique that detects specific DNA-sequences. It is highly sensitive and accurate for identifying residual host cell DNA contaminants in biology. In host cell contaminant testing, it is essential to enable precise detection and quantification of bacteria, viruses, and fungi in biology.
- In 2022, Bruker launched FluoroType® Mycobacteria PCR assay. It differentiates 31 clinically relevant non-tuberculous mycobacteria and the M. tuberculosiscomplex from cultures in host cell contaminant testing. FluoroType® Mycobacteria PCR assay uses LiquidArray® technology which incorporates innovative probe and software read-outs.
Therefore, rising adoption of advanced PCR-based assay is leading to host cell contaminant testing market demand by enhancing sensitivity and identification of residual host cell DNA contaminants.
Growing Incorporation of Biologics in the Treatment of Chronic Diseases is creating Host Cell Contaminant Testing Market Demand
Biologics are medical products derived from living organisms which includes vaccines, gene therapies, monoclonal antibodies, and others. They provide affordable treatment options for chronic diseases such as cancer, diabetes, and rheumatoid arthritis, reducing healthcare costs. Host cell contaminant testing is essential in biologics production for detecting and quantifying residual contaminants in host cells.
- In 2021, according to American Chemical Society, global biologics market is expected to grow by 9.2% by the year 2027. Host cell contaminant testing is essential in biologics development & production as it identifies & quantifies residual host cell contaminants which ensures the safety and efficacy of biologics.
Hence, growing incorporation of biologics in the chronic diseases treatment is leading to host cell contaminant testing market expansion by ensuring safety and efficacy in the development and production of biologics.
Key Restraints:
Stringent Regulatory Approvals are constraining Host Cell Contaminant Testing Market Growth
Regulatory approvals are a significant restrain in the market demand due to stringent requirements for the approval of biologics. Some countries have unique requirements for host cell contaminant testing protocols including acceptable thresholds for residual proteins, validations processes and reporting standards. These validations delay the approval of biologics and biosimilar, as companies must adopt their testing method to meet region-specific criteria. Regulatory agencies like United States Food and Drug Administration, European Medicines Agency, and others enforce stringent quality standards to ensure the safety and efficacy of biologics. Additionally, regulations requires huge investment in compliance sources, adding to operational cost.
Thus, regulatory approvals are constraining host cell contaminant testing market expansion by increasing the time and operational cost for the host cell contaminant testing providers.
Future Opportunities :
Development of Direct Sandwich Enzyme-Linked Immunosorbent Assay Kit is expected to create Host Cell Contaminant Testing Market Opportunities
Direct sandwich ELISA kit is a type of enzyme-linked immunosorbent assay which is designed to detect and quantify specific antigens, typically proteins in a sample. It is essential in host cell contaminant testing to detect and quantify host cell proteins in the biologics manufacturing. By enabling accurate host cell protein detection, direct sandwich ELISA kit facilitates the production of safer and more effective biologics.
- In 2024, Krishgen Biosystems launched KRIBIOLISA™ High Five (H5) host cell protein ELISA kit. This kit is designed with a direct sandwich assay format to facilitate accurate and efficient detection of CRM197 protein in host cell contaminant testing. It enables better specificity and sensitivity compared to conventional ELISA kits.
Thus, development of direct sandwich ELISA kit is expected to create host cell contaminant testing market opportunities by facilitating accurate and efficient detection of proteins in host cells.
Host Cell Contaminant Testing Market Segmental Analysis :
By Test:
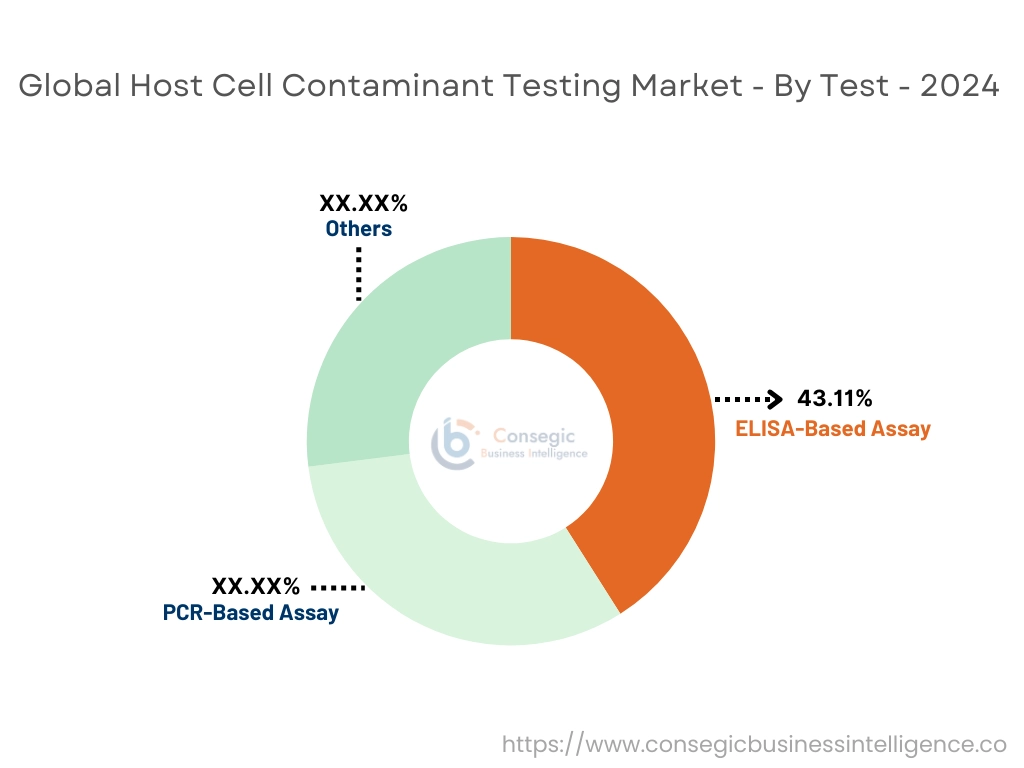
By test, the market is divided into ELISA-based assay, PCR-based assay and others.
Trends in Test:
- According to host cell contaminant testing market trends, ELISA-based assay is widely used due to their high accuracy and detection of wide range of contaminants.
- Adoption of PCR-based assay is growing to detect host cell DNA contaminants in biology.
The ELISA-based assay accounted for the largest market share of 43.11% in the year 2024.
- The ELISA-based assay is a biochemical technique used to detect and quantify specific proteins or antigens by using colorimetric and fluorescent readout system.
- The ELISA-based assay is the most widely used host cell contaminant testing due to its high sensitivity, specificity, and accuracy.
- It relies on polyclonal antibodies raised against host cell proteins which enables the simultaneous detection of wide range of contaminants.
- Further, companies are investing in the development of ELISA-based assay kit for reliable quantification during the development and manufacturing process of biopharmaceuticals.
- In 2022, Rockland Immunochemicals, Inc. and Cytiva Life Sciences launched PrismA ELISA Kit for the development and manufacture of monoclonal antibody biopharmaceuticals. It provides high capacity and consistent purification performance in host cell contaminant testing.
- Thus, ELISA-based assay is dominating in the market due to high accuracy and detection of wide range of contaminants.
The PCR-based assay is expected to grow at the fastest CAGR over the forecast period.
- A PCR-based assay is a molecular technique that detects specific DNA-sequences, and it is highly sensitive and accurate for identifying residual host cell DNA contaminants in biologics.
- The use of this assay is growing in host cell contaminant testing due to high sensitivity and specificity which leads to precise quantification of even minute DNA contaminants from host cells such as E. coli.
- Factors such as increasing demand for biologics, stringent regulatory requirements for DNA contamination limits, and the adoption of automated PCR systems are driving the use of PCR-based assay in the market.
- Thus, use of PCR-based assay is growing in the market due to high sensitivity and detection accuracy as per market trends.
By Type:
By type, the market is divided into mammalian, microbial, and others.
Trends in Type:
- As per host cell contaminant testing market trends, mammalian testing is widely used to detect and quantify residual contaminants due to high specificity and accuracy.
- Microbial testing is increasingly adopted in the market for detection and identification of microorganisms such as bacteria, fungi, and viruses in biologics as per trends.
The mammalian accounted for the largest market share in the year 2024.
- Mammalian host cell contaminant testing refers to the detection and quantification of residual contaminants, such as host cell proteins and DNA.
- These contaminants must be minimized to ensure the safety and efficacy of biologics like monoclonal antibodies, recombinant proteins, and others.
- The ELISA-based assay is widely used in mammalian host cell contaminant testing due to its high sensitivity, specificity, and accuracy.
- DNA testing is also preferred especially due to regulatory compliance imposing stringent limits on allowable DNA levels in biologics, further contributing to the market demand.
- Thus, mammalian host cell contaminant testing is dominant in the market due to high specificity and accuracy.
The microbial is expected to grow at the fastest CAGR over the forecast period.
- Microbial host cell contaminant testing refers to the detection and identification of microorganisms such as bacteria, fungi, and viruses that will contaminate biologics.
- It ensures that products are free from harmful microbial contaminants to meet safety and regulatory standards.
- The PCR-based assay is widely used in microbial host cell contaminant testing due to high sensitivity, specificity and ability to provide rapid results.
- Further, companies are investing in the development of PCR-based assay kit for reliable quantification of viruses during microbial testing in biologics.
- For instance, in 2024, Erba Transasia launched MonkeyPox PCR based assay kit which is approved by Central Drugs Standard Control Organization. It delivers 97.5% sensitivity and 100% specificity and detects Monkey Pox virus in the host cell contaminant testing.
- Therefore, microbial host cell contaminant testing is expanding in the market due to regulatory standards and high detection accuracy as per market trends.
By End Use:
By end-user, the market is divided into pharmaceutical & biopharmaceutical companies, contract research organizations, contract manufacturing organizations, and others.
Trends in End-User:
- According to market trends, pharmaceutical and biopharmaceutical companies widely used host cell contaminant testing in the development of therapy drugs and vaccines.
- Contract research organizations are rapidly adopting host cell contaminant testing for quality control processes in the biologics manufacturing as per market trends.
The pharmaceutical and biopharmaceutical companies accounted for the largest market share in the year 2024.
- The pharmaceutical and biopharmaceutical companies are comprised of organizations engaged in researching, developing, manufacturing, and distributing drugs.
- Host cell contaminant testing is widely used by these companies to detect and remove residual host cell contaminants that will impact drug safety and stability.
- It also ensures the safety and efficacy of biologics such as monoclonal antibodies, vaccines, and biosimilars.
- Further, companies are investing in digital PCR assay for precise detection and high accuracy in host cell contaminant testing.
- For instance, in 2024, QIAGEN launched digital PCR assay for use on its digital PCR platform QIAcuity. It offers a wide range of assay customization options, from simple to complex and validated multiplex assays in host cell contaminant testing.
- Thus, pharmaceutical and biopharmaceutical companies widely incorporate host cell contaminant testing for drug safety and stability.
The contract research organizations are expected to grow at the fastest CAGR over the forecast period.
- A contract research organization is a company that provides support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis.
- Contract research organizations are increasingly adopting host cell contaminant testing for biologics manufacturing companies that outsource critical quality control processes.
- The growing complexity of host cell contaminant testing coupled with the need for compliance with stringent regulatory standards are encouraging companies to rely on contract research organization for their expertise and advanced testing capabilities.
- Additionally, contract research organization helps to reduce operational costs and time in the production of biologics further driving the market.
- Thus, contract research organizations are widely adopting host cell contaminant testing in the production of biologics.
Regional Analysis:
The regional segment includes North America, Europe, Asia Pacific, the Middle East and Africa, and Latin America.
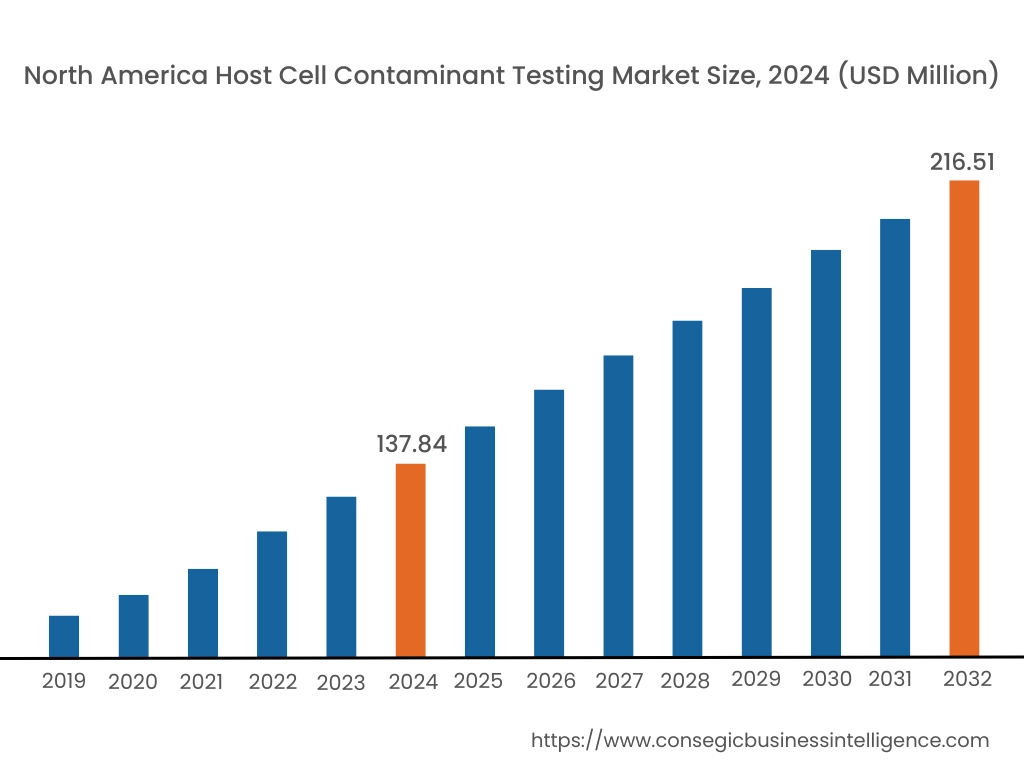
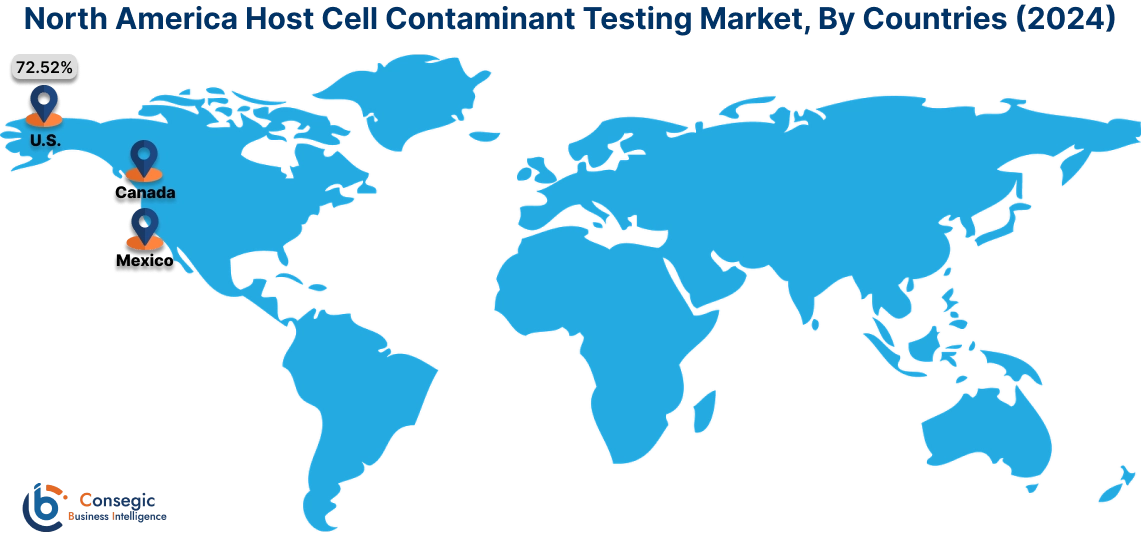
In 2024, North America accounted for the highest market share at 43.61% and was valued at USD 137.84 Million and is expected to reach USD 216.51 Million in 2032. In North America, United States accounted for the highest market share of 72.52% during the base year of 2024.
The host cell contaminant testing market share of North America is significant due to strong presence of pharmaceutical companies, regulatory bodies, and advanced research facilities. The clinical research companies in the region are investing in the development of advanced digital PCR assays, further boosting the market.
- In 2024, Bio-Rad launched multiplexed digital PCR assay for breast cancer mutation detection in tissue samples. It is available with analytical sensitivity down to 0.01% variant allele fraction which gives same day results in host cell contaminant testing.
Thus, North America is leading in the market due to growing investment in research facilities and strong presence of pharmaceutical companies as per analysis.
Asia-pacific is expected to witness the fastest CAGR of 7.7% over the forecast period during 2025-2032.
As per host cell contaminant testing market analysis, the Asia-pacific region is experiencing rapid growth in the market fueled by rising investments in biotechnology and expanding biopharmaceutical industry. Countries like China, India, and Japan are experiencing rapid growth in biologics and biosimilar production driving demand for host cell contaminant testing. The regulatory agencies in the region are widely adopting advanced host cell contaminant testing methods like digital PCR, further driving the market. Hence, the host cell contaminant testing market share of Asia-Pacific is expanding due to expanding biotechnology sector and increasing biosimilar production as per analysis.
According to host cell contaminant testing market analysis, Europe region is experiencing considerable growth in the market driven by presence of stringent regulatory bodies and strong biopharmaceutical sector. The regulatory agencies like European Medicines Agency are incorporating host cell contaminant testing to ensure the safety, quality, and efficacy of biosimilar, biologics, and vaccines. Additionally, the growing focus on personalized medicine and investments in biosimilar development is further driving the market in the region.
The Middle East and Africa region is experiencing gradual growth in the market, driven by expanding biopharmaceutical manufacturing and increasing investments in the healthcare infrastructure. The countries like South Africa, UAE and Saudi Arabia are investing in host cell contaminant testing to ensure regulatory compliance and product safety. Additionally, the outsourcing of host cell contaminant testing to contract research organization is growing, further leading to host cell contaminant testing market proliferation according to market analysis.
As per market analysis, the host cell contaminant testing market in Latin America is driven by increasing investment in research and growing biosimilar production. Countries like Brazil, Mexico, and Argentina are leading in the market due to their robust pharmaceutical sectors and research facilities. Additionally, the rise of contract research organizations in the region is promoting the outsourcing of host cell protein testing, further contributing to the market growth.
Top Key Players and Market Share Insights:
The host cell contaminant testing industry is highly competitive with major players providing services to the national and international markets. Key players are adopting several strategies in research and development (R&D) and product innovation to hold a strong position in the global host cell contaminant testing market. Key players in the host cell contaminant testing industry include-
- Thermo Fisher Scientific (United States)
- Bio-Rad Laboratories, Inc. (United States)
- Cytiva (United States)
- Enzo Life Sciences Inc. (United States)
- Biogenes GmbH (Germany)
- BioGenes GmbH (Germany)
- Agilent Technologies, Inc. (United States)
- Cisbio Bioassays (France)
- Cygnus Technologies LLC (United States)
- Charles River Laboratories (United States)
Recent Industry Developments :
Product Launches:
- In 2023, Gold Standard Diagnostics launched DNAllergen real-time PCR- based assay kits. It enables the detection of some specific allergens in host cell contaminant testing which are not easy to identify by immunoassays.
Partnerships & Collaborations:
- In 2021, Bio-Rad Laboratories, Inc. collaborated with Seegene, Inc., for the clinical development and commercialization of infectious disease molecular diagnostic products. It resulted in multiplex real-time PCR detection and differentiation with high sensitivity and specificity in host cell contaminant testing.
Host Cell Contaminant Testing Market Report Insights :
| Report Attributes | Report Details |
| Study Timeline | 2019-2032 |
| Market Size in 2032 | USD 521.77 Million |
| CAGR (2025-2032) | 6.5% |
| By Test |
|
| By Type |
|
| By End User |
|
| By Region |
|
| Key Players |
|
| North America | U.S. Canada Mexico |
| Europe | U.K. Germany France Spain Italy Russia Benelux Rest of Europe |
| APAC | China South Korea Japan India Australia ASEAN Rest of Asia-Pacific |
| Middle East and Africa | GCC Turkey South Africa Rest of MEA |
| LATAM | Brazil Argentina Chile Rest of LATAM |
| Report Coverage |
|
Key Questions Answered in the Report
How big is the host cell contaminant testing market? +
In 2024, the host cell contaminant testing market is USD 316.08 million.
Which is the fastest-growing region in the host cell contaminant testing market? +
Asia-Pacific is the fastest-growing region in the host cell contaminant testing market.
What specific segmentation details are covered in the host cell contaminant testing market? +
Test, type and end-user are covered in the host cell contaminant testing market.
Who are the major players in the host cell contaminant testing market? +
Thermo Fisher Scientific (United States), Bio-Rad Laboratories, Inc. (United States), and BioGenes GmbH (Germany) are some of the major players in the market.