HIV Diagnostics Market Size:
HIV Diagnostics Market size is growing with a CAGR of 6.6% during the forecast period (2024-2031), and the market is projected to be valued at USD 6,128.95 Million by 2031 from USD 3,701.57 Million in 2023.
HIV Diagnostics Market Scope & Overview:
HIV stands for Human Immunodeficiency Virus. It is a virus that attacks the body's immune system, specifically targeting CD4 cells, which are crucial for fighting off infections. HIV diagnostics involves a series of examinations to detect the presence of HIV antibodies or the virus itself in the body. Initially, rapid tests provide quick, preliminary results. These tests detect HIV antibodies, which the body produces in response to the virus. If rapid tests are positive, they are followed by confirmatory tests, such as ELISA (Enzyme-Linked Immunosorbent Assay) or Western blot, which provide more accurate results. ELISA detects HIV antibodies by using specific enzymes to identify them. Western blot is a more complex test that identifies specific HIV proteins. In addition to antibody tests, nucleic acid tests (NATs) directly detect the virus's genetic material. These tests are highly sensitive and detect HIV infection in the early stages before antibody tests become positive. Additionally, various software and instruments are introduced for the detection of HIV. The end-user for diagnostics of HIV includes hospitals & clinics, diagnostic laboratories, and home care settings.
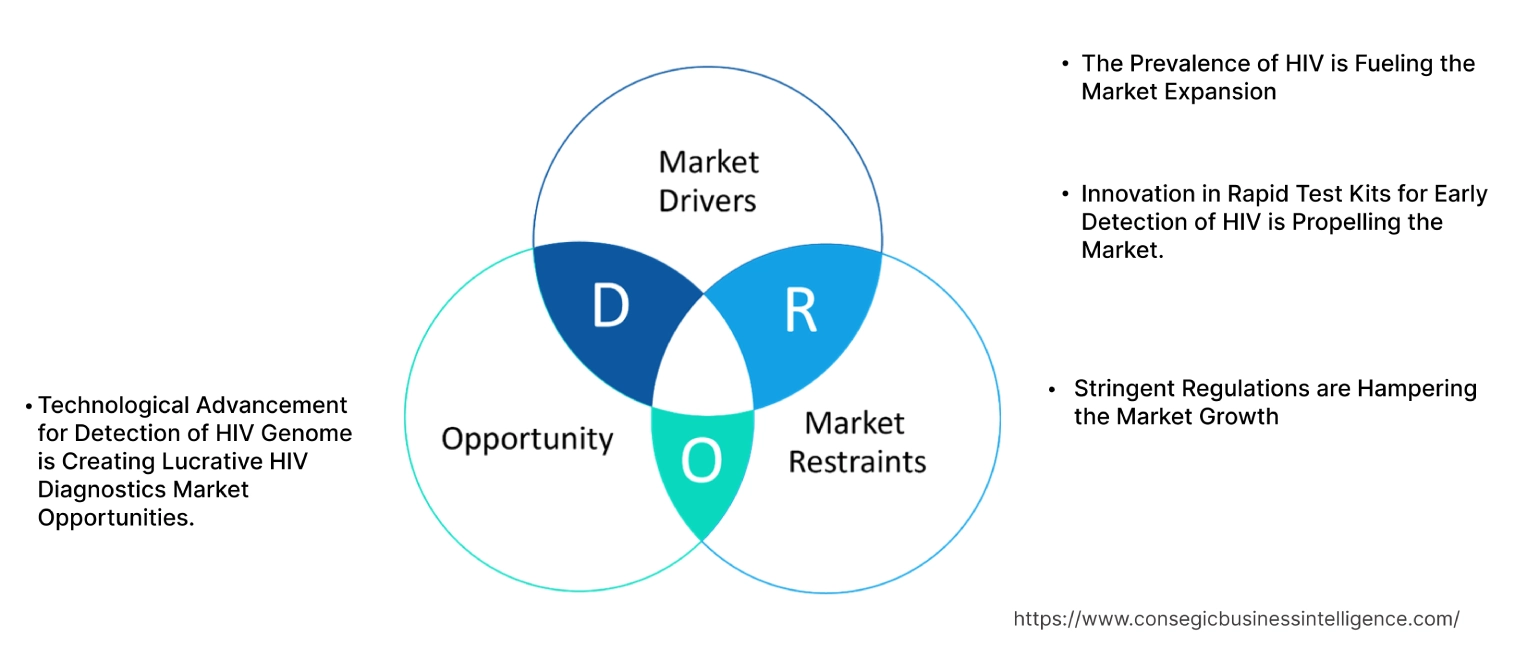
HIV Diagnostics Market Dynamics - (DRO) :
Key Drivers:
The Prevalence of HIV is Fueling the Market Expansion.
The pervasive presence of HIV continues to fuel the growth of the market. The escalating number of HIV infections worldwide necessitates robust and accessible diagnostic tools. The persistence of HIV is due to a combination of factors.
Unsafe sexual practices, including unprotected sex and multiple partners, remain a primary mode of transmission. Additionally, sharing needles and syringes among intravenous drug users is another significant risk factor. Additionally, mother-to-child transmission occurs during pregnancy, childbirth, or breastfeeding if proper preventive measures are not taken. These factors lead to the prevalence of HIV.
For instance,
- According to the data published by the World Health Organization, in 2023, an estimated 1.3 million individuals contracted HIV globally. As of the end of 2023, approximately 39.9 million people were living with HIV, including 1.4 million children and 38.6 million adults.
Hence, the prevalence of HIV is influencing the need for accurate and timely diagnosis for initiating appropriate treatment, preventing further transmission, and improving patient outcomes, thus driving the HIV diagnostics market growth.
Innovation in Rapid Test Kits for Early Detection of HIV is Propelling the Market.
Innovations in diagnostic kits for HIV are significantly driving the market expansion. These innovative kits offer rapid, accurate, and user-friendly solutions, empowering individuals to take control of their health and seek timely medical intervention.
The development of these kits is driven by the need for early diagnosis to prevent further transmission and initiate effective treatment. By improving accessibility and reducing the time to results, these tests are playing a crucial role in expanding HIV testing services, particularly in remote areas. Manufacturers are introducing novel diagnostic kits that are easy to use.
For instance,
- In 2023, Mylab Discovery Solutions launched three new rapid test kits for the early detection of HIV, HCV, and Syphilis. These tests are designed to be easy to use, storable at room temperature, and deployable in point-of-care settings, including blood banks. This innovation aims to improve accessibility to testing.
Thus, the surge in early detection and diagnosis is expected to propel the growth of the market.
Key Restraints :
Stringent Regulations are Hampering the Market Growth.
Stringent regulations imposed by regulatory bodies such as the Food and Drug Administration and the European Medicines Agency significantly hamper the growth of the market. These regulations, while essential for ensuring product safety and efficacy, often involve lengthy and complex approval processes, including clinical trials and extensive documentation. This delays the market entry of innovative diagnostic technologies, limiting access to advanced testing methods, particularly in resource-limited settings.
Additionally, stringent quality control standards and manufacturing regulations increase the production costs of instruments and software, making diagnostic tests less affordable, especially in developed countries. These regulations, while essential for ensuring the accuracy and reliability of diagnostic tests, lead to higher manufacturing costs. This limits access to advanced diagnostic technologies in regions with limited healthcare budgets, hindering early detection and treatment of HIV. Thus, based on the analysis, the aforementioned factors are hindering the HIV diagnostics market growth.
Future Opportunities :
Technological Advancement for Detection of HIV Genome is Creating Lucrative HIV Diagnostics Market Opportunities.
Technological advancements in HIV genome detection are creating lucrative HIV diagnostics market opportunities. The development of innovative diagnostic techniques, such as molecular diagnostics amongst others, has significantly improved the accuracy, sensitivity, and speed of HIV testing. These advancements enable earlier detection of HIV infections, facilitating timely initiation of treatment, and reducing the risk of transmission. Moreover, the development of point-of-care testing (POCT), which is a diagnostic test, particularly for patient care made HIV testing more accessible, especially in resource-limited settings. Various research activities have led to the development of novel technologies for the detection of the HIV genome.
For instance,
- According to the data published by the Press Information Bureau of India, in 2024, researchers developed a new fluorometric test to accurately detect HIV-genome derived G-Quadruplex (GQ), a unique four-stranded DNA structure. This innovative diagnostic platform aims to significantly reduce false positive HIV test results, offering a more reliable and accurate diagnosis.
As per the HIV diagnostics market analysis, as technology continues to evolve, novel tests are introduced leading to improved patient outcomes and a more effective global response to HIV in the coming years.
HIV Diagnostics Market Segmental Analysis :
By Type:
Based on type, the market is categorized into instruments, and software & services.
Trends in the Type:
- Advanced molecular techniques and rapid diagnostic tests are improving the accuracy of HIV tests.
- Self-testing kits are gaining popularity, offering privacy and convenience for individuals who are hesitant to visit a healthcare facility.
The instruments segment accounted for the largest HIV diagnostics market share in 2023.
- HIV diagnostics rely on a variety of instruments to detect the virus or antibodies against it.
- Rapid diagnostic tests (RDTs) are simple, point-of-care tests that provide results within minutes. These tests detect HIV antibodies in blood or oral fluid. For more accurate and sensitive testing, enzyme-linked immunosorbent assays (ELISAs) are used to detect HIV antibodies in blood samples.
- Nucleic acid tests (NATs) detect HIV genetic material, allowing for earlier diagnosis. These tests often involve sophisticated laboratory equipment, such as real-time PCR machines, to amplify and detect viral DNA or RNA.
- Moreover, diagnostic kits are essential tools in HIV diagnostics, providing a convenient and efficient way to detect the virus. These kits typically contain all the necessary reagents and materials for conducting the test.
- Various manufacturers are introducing novel instruments such as kits for HIV diagnostic because of the rise in demand for these solutions.
- For instance, in 2022, Thermo Fisher Scientific, a leading provider of scientific research solutions, launched an enhanced version of its Applied Biosystems HIV-1 Genotyping Kit. This advanced kit offers improved sensitivity and accuracy in detecting and characterizing HIV-1 viral load and drug resistance mutations.
- By utilizing these instruments, healthcare providers accurately diagnose HIV infections and monitor disease progression, thus influencing the HIV diagnostics market trends.
The software & services segment is expected to grow at the fastest CAGR over the forecast period.
- Software & services play a crucial role in the field of HIV diagnostics.
- Specialized software is used to analyze and interpret test results, ensuring accuracy and efficiency.
- These software solutions significantly enhance the efficiency and accuracy of HIV diagnostics. By automating data entry, these tools reduce manual effort and minimize the risk of human error.
- They enable efficient tracking of patient information, including test results, treatment history, and viral load monitoring.
- Additionally, HIV diagnostic services involve a range of tests to detect the presence of HIV in the body.
- These tests are conducted through blood or saliva samples and typically involve antibody tests, which look for antibodies produced by the immune system in response to the virus. Rapid tests provide results within minutes, while more complex tests may require laboratory analysis. Early detection is crucial for timely treatment and prevention of HIV transmission.
- Furthermore, cloud-based platforms enable remote access to patient data, facilitating collaboration between healthcare providers and improving the overall efficiency and demand for HIV testing.
- Thus, as per the analysis, the utilization of software & services is influencing the HIV diagnostics market expansion.
By End-User:
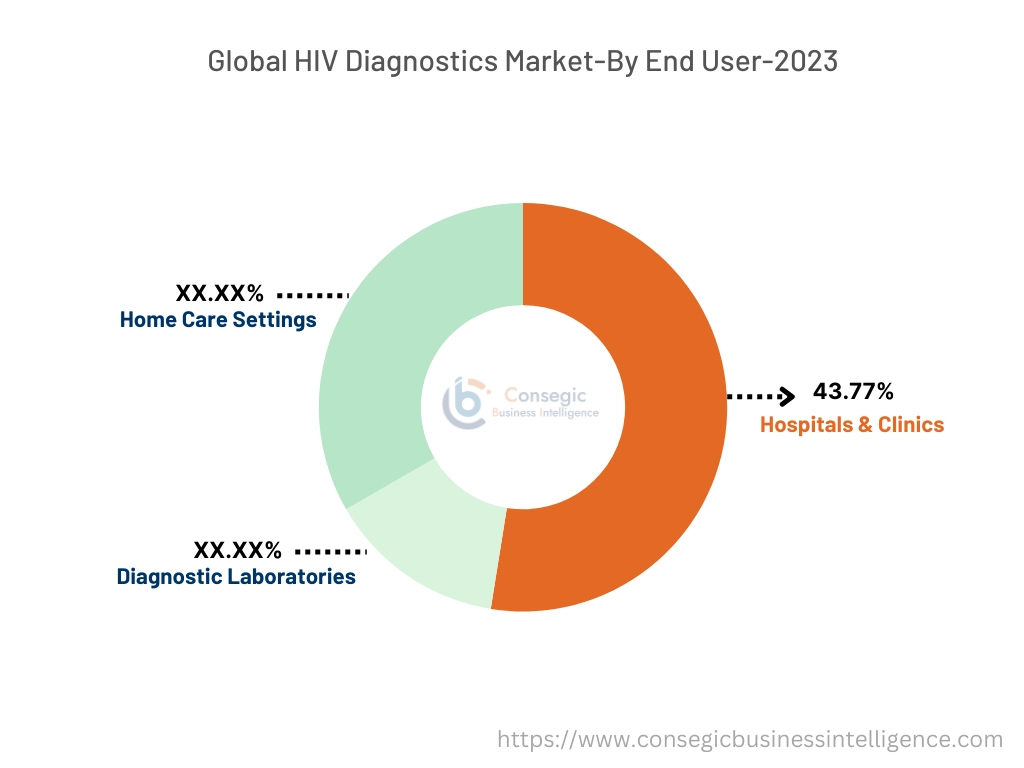
Based on end users, the market is categorized into hospitals & clinics, diagnostic laboratories, and home care settings.
Trend in the End User:
- Diagnostic Laboratories are adopting advanced technologies, such as nucleic acid amplification tests (NAATs), to improve diagnostic accuracy and sensitivity, especially in early-stage infections.
The hospitals & clinics segment accounted for the largest HIV diagnostics market share of 43.77% in the year 2023.
- HIV diagnostics in hospitals and clinics involve a multi-step process. Initial screening often utilizes rapid antibody tests that provide quick results.
- Positive rapid tests are typically confirmed with more sensitive laboratory-based tests like ELISA (Enzyme-Linked Immunosorbent Assay) to detect HIV antibodies.
- For definitive diagnosis, Western blot tests are performed to identify specific HIV proteins.
- In addition to antibody tests, nucleic acid tests (NATs) directly detect the virus's genetic material, enabling early diagnosis, especially in individuals with recent infections.
- Hospitals and clinics often offer counseling and support services alongside testing to ensure informed decision-making and adherence to treatment.
- Thus, the aforementioned factors are driving the market trends
The homecare settings segment is expected to grow at the fastest CAGR over the forecast period.
- Homecare settings are increasingly becoming prominent for HIV diagnostics. As healthcare shifts towards patient-centered care, home-based testing offers several advantages.
- Homecare settings help reduce the burden on healthcare facilities, particularly in resource-limited settings.
- These settings alleviate the pressure on clinics and hospitals, allowing them to focus on more complex cases.
- Moreover, homecare settings offer privacy and confidentiality to individuals, reducing the concerns associated with HIV testing.
- This encourages more people to seek testing, leading to increased early diagnosis and improved health outcomes.
- Manufacturers are introducing novel solutions for diagnostics of HIV in homecare settings.
- For instance, in 2024, The Ministry of Health (MoH) launched a new self-testing kit to bolster the fight against HIV/AIDS, making it more accessible for individuals to test themselves in the privacy of their homes. This innovative approach empowers people to take control of their health and seek timely medical care. By providing convenient and confidential testing options, the MoH aims to increase early diagnosis and reduce the spread of HIV.
- Thus, the incorporation of diagnostic solutions for HIV in homecare settings is influencing the HIV diagnostics market demand and trends in the coming years.
Regional Analysis:
The regional segment includes North America, Europe, Asia Pacific, the Middle East and Africa, and Latin America.
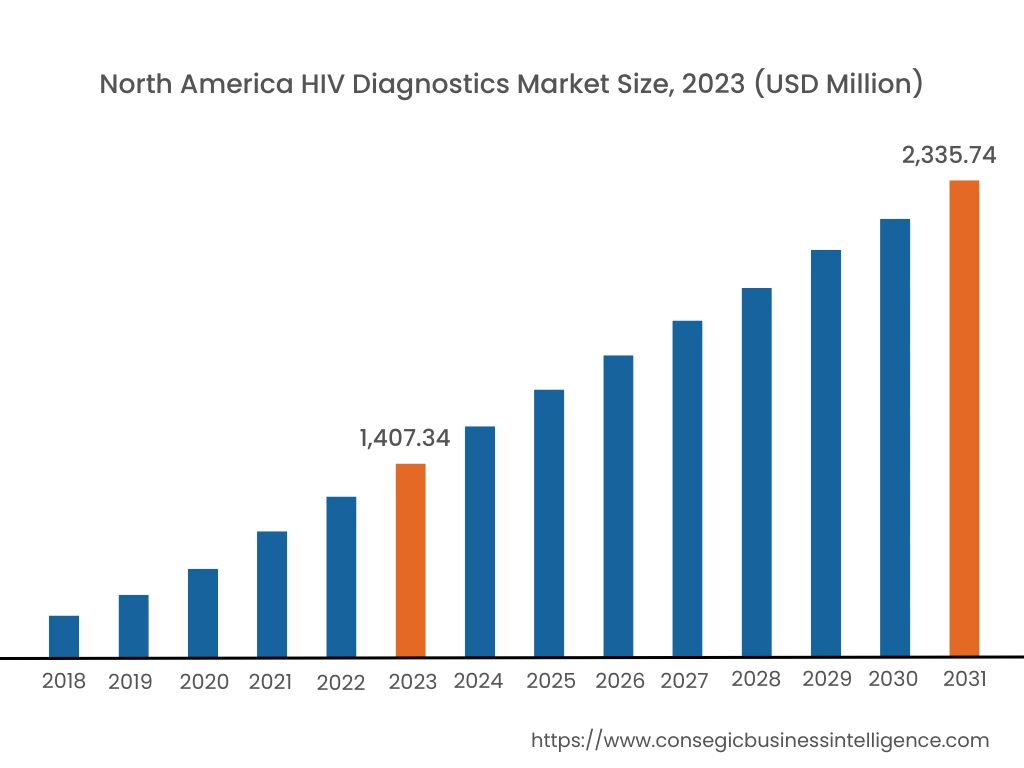
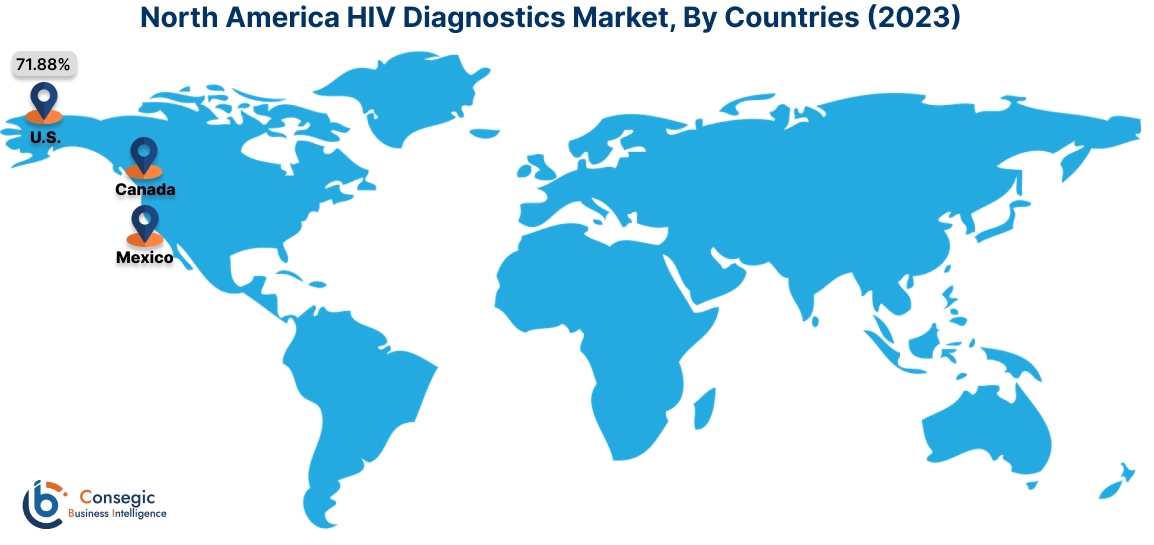
In 2023, North America accounted for the highest market share at 38.02% and was valued at USD 1,407.34 Million and is expected to reach USD 2,335.74 Million in 2031. In North America, the U.S. accounted for the highest market share of 71.88% during the base year of 2023.
HIV diagnostics in North America have significantly advanced, with a focus on early detection, improved accuracy, and patient-centered care. Rapid diagnostic tests (RDTs) have become widely available, allowing for quick and convenient testing in various settings, including healthcare facilities and community-based organizations. These RDTs detect HIV antibodies in blood or oral fluid, providing results within minutes. Additionally, nucleic acid tests (NATs) are used to detect HIV genetic material, enabling earlier diagnosis and improving treatment outcomes. Advanced laboratory techniques, such as enzyme-linked immunosorbent assays (ELISAs), are employed to confirm HIV infections and monitor disease progression. These technological advancements, coupled with increased awareness and access to testing, have contributed to a decline in new HIV infections and improved the quality of life for individuals living with HIV in North America. As per the HIV diagnostics market analysis, the prevalence of HIV in North American countries is influencing the rise in diagnosis and the use of diagnostic solutions.
For instance,
- According to the data published by the US government, in 2022, approximately 31,800 people in the U.S. contracted HIV.
Thus, the aforementioned factors are driving the HIV diagnostics market trends in North America.
The Asia Pacific market for HIV Diagnostics is experiencing the fastest growth with a CAGR of 6.9 % over the forecast period. The Asia Pacific region has made significant strides in HIV diagnostics, leading to improved outcomes for individuals living with HIV. Increased access to testing facilities, particularly in urban areas, has facilitated early diagnosis. The development and widespread use of rapid diagnostic tests have enabled quicker results, allowing for the timely initiation of treatment. Additionally, advancements in laboratory technologies have enhanced the accuracy and sensitivity of HIV testing, improving the overall quality of care. Overall, these positive developments and trends have contributed to a substantial decline in HIV-related morbidity and mortality in the region, significantly improving the health and quality of life for individuals living with HIV in the Asia Pacific region.
HIV diagnostics in Europe have significantly advanced, enabling early detection and timely treatment of HIV/AIDS. Rapid tests and nucleic acid tests (NAT) are widely accessible in healthcare settings, facilitating swift diagnosis. Early detection has dramatically improved the quality of life for people living with HIV, reducing morbidity and mortality rates. Furthermore, widespread testing campaigns and public awareness programs have empowered individuals to make informed decisions about their sexual health, leading to a decline in new HIV infections. The integration of HIV testing into routine healthcare services, coupled with confidential counseling and support, has fostered a more inclusive and supportive healthcare environment for individuals living with HIV, thus driving the trends for market growth.
HIV diagnostics in the Middle East and Africa have been essential in addressing the significant burden of HIV/AIDS. Significant progress has been made in recent years. The implementation of point-of-care rapid tests has facilitated testing in remote areas, while increased availability of laboratory-based tests has improved diagnostic accuracy. Additionally, public health initiatives and awareness campaigns have helped to reduce stigma and encourage people to seek testing. Ongoing efforts to strengthen healthcare infrastructure, improve access to testing, and promote early treatment are crucial in controlling the spread of HIV and improving the lives of people living with HIV in the Middle East and Africa. Thus, these factors are influencing the HIV diagnostics market demand.
HIV diagnostics in Latin America have significantly contributed to the region's efforts to combat HIV. Advanced testing methods, including rapid tests and nucleic acid tests (NAT), have facilitated early detection and timely treatment. Additionally, widespread testing campaigns and public awareness programs have empowered individuals to make informed decisions about their sexual health, leading to a decline in new HIV infections. The rise in the demand for HIV testing in routine healthcare services, coupled with confidential counseling and support, has fostered a more inclusive and supportive healthcare environment for individuals living with HIV. Thus, these factors are further influencing the HIV diagnostics market expansion.
Top Key Players & Market Share Insights:
The HIV Diagnostics industry is highly competitive with major players providing precise measurements between objects to the national and international markets. Key players are adopting several strategies in research and development (R&D) and product innovation to hold a strong position in the global HIV Diagnostics market. Key players in the HIV Diagnostics industry include-
- Abbott (United States)
- Meril Life Sciences Pvt. Ltd. (India)
- Wantai BioPharm (China)
- QIAGEN (Germany)
- OraSure Technologies Inc.(United States)
- Bio-Rad Laboratories, Inc (United States)
- Hologic, Inc. (United States)
- Siemens Healthcare Private Limited (India)
- Beckman Coulter, Inc. (United States)
Recent Industry Developments :
Laboratory Launches:
- In 2023, Y Kikheto Sema, IAS Commissioner & Secretary H&FW and Chairman NSACS, inaugurated Nagaland's first HIV-1 Viral Load Laboratory at Naga Hospital Authority Kohima.
Product Launches:
- In November 2024, The Federal Government launched a novel toolkit to develop Early Infant Diagnosis (EID) services in Taraba and Rivers states. Developed in collaboration with the Elizabeth Glaser Pediatric AIDS Foundation (EGPAF), this initiative aims to optimize EID programs by addressing critical gaps and implementing evidence-based strategies. By prioritizing early diagnosis and treatment, the government is taking proactive steps to reduce the burden of HIV infection among infants and children, ensuring a healthier future for generations to come.
HIV Diagnostics Market Report Insights :
| Report Attributes | Report Details |
| Study Timeline | 2018-2031 |
| Market Size in 2031 | USD 6,128.95 Million |
| CAGR (2024-2031) | 6.6% |
| By Type |
|
| By End-User |
|
| By Region |
|
| Key Players |
|
| North America | U.S. Canada Mexico |
| Europe | U.K. Germany France Spain Italy Russia Benelux Rest of Europe |
| APAC | China South Korea Japan India Australia ASEAN Rest of Asia-Pacific |
| Middle East and Africa | GCC Turkey South Africa Rest of MEA |
| LATAM | Brazil Argentina Chile Rest of LATAM |
| Report Coverage |
|
Key Questions Answered in the Report
How big is the HIV Diagnostics market? +
In 2023, the HIV Diagnostics market is USD 3,701.57 million.
Which is the fastest-growing region in the HIV Diagnostics market? +
Asia Pacific is the fastest-growing region in the HIV Diagnostics market.
What specific segmentation details are covered in the HIV Diagnostics market? +
Type and End-user segmentation details are covered in the HIV Diagnostics market.
Who are the major players in the HIV Diagnostics market? +
Abbott (United States), Meril Life Sciences Pvt. Ltd. (India), Wantai BioPharm (China), Bio-Rad Laboratories, Inc. (United States), Hologic, Inc. (United States), Siemens Healthcare Private Limited (India), Beckman Coulter, Inc. (United States), OraSure Technologies Inc.(United States), QIAGEN (Germany).