- Summary
- Table Of Content
- Methodology
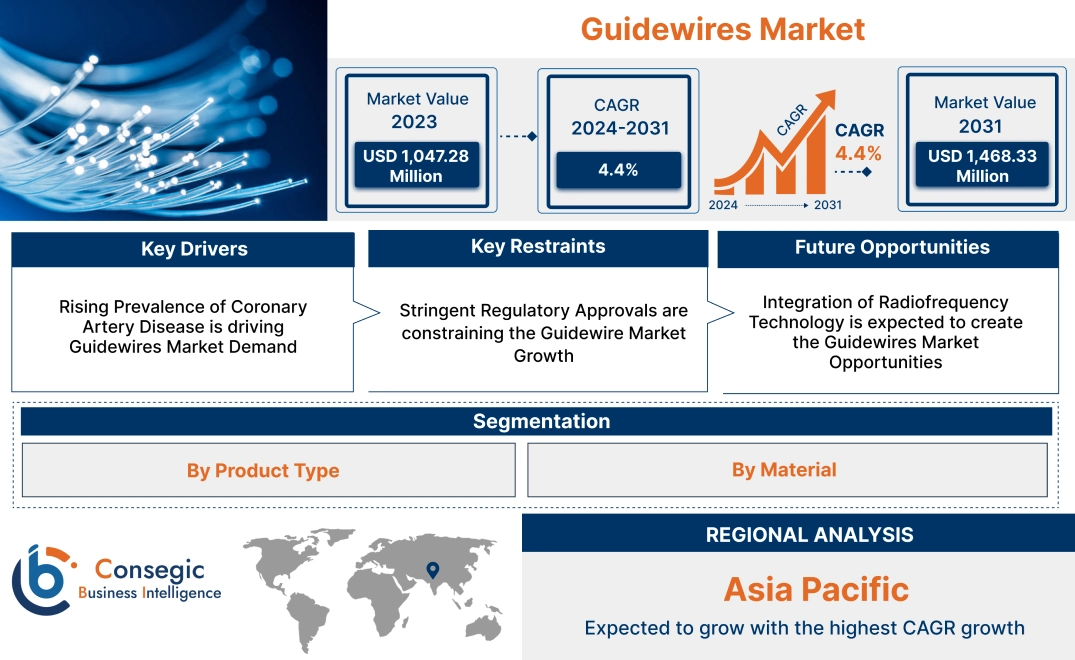
Guidewires Market Size:
Guidewires market size is estimated to reach over USD 1,468.33 Million by 2031 from a value of USD 1,047.28 Million in 2023, growing at a CAGR of 4.4% from 2024 to 2031.
Guidewires Market Scope & Overview:
The guidewires are thin, flexible wires used to guide needles, tubes, or catheters through blood vessels. The main characteristics of the guidewires are flexibility, torque control, and others. Their purpose is to gain access to the blood vessels using a minimally invasive technique. They are used to access the coronary and peripheral vascular systems. Their application is in various procedures such as angioplasty, valve replacement procedures, carotid artery interventions, and others. There are different types of guidewires such as coronary, peripheral, neurovascular, and others. They are made up of various materials such as stainless steel, nitinol, and others. Recent developments such as artificial intelligence-enabled guidewires, and radiofrequency-enabled guidewires are driving the market.
Guidewires Market Dynamics - (DRO) :
Key Drivers:
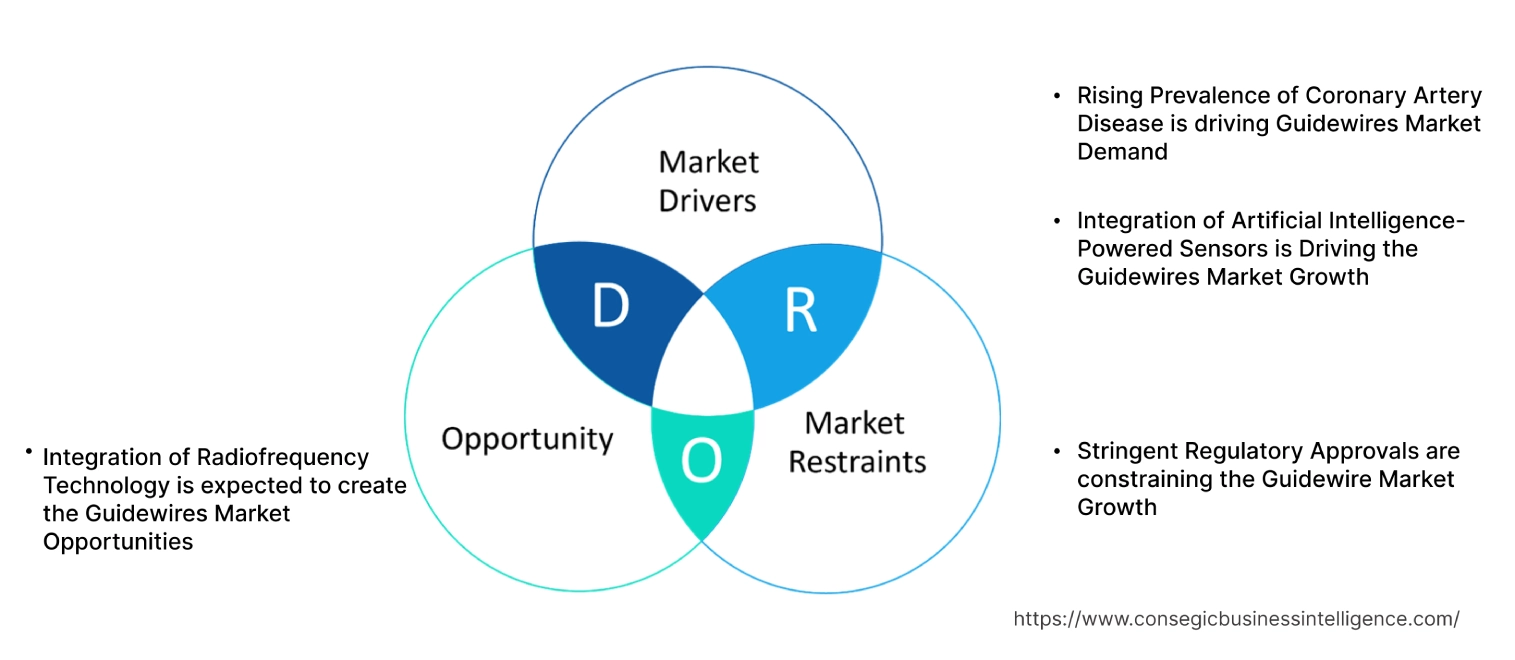
Rising Prevalence of Coronary Artery Disease is driving Guidewires Market Demand
Coronary artery disease (CAD) is characterized by the narrowing or blockage of coronary arteries due to plaque buildup, leading to reduced blood flow to the heart. This condition typically requires minimally invasive interventions such as angioplasty, where guidewires are necessary to navigate the catheter to gain access to blocked vessels and placing of stents. The cases of CAD are rising due to the growing aging population, sedentary lifestyle, and other factors.
- In 2022, according to a research paper published in the Journal of the American College of Cardiology, there are 315 million prevalent cases of coronary artery disease (CAD) globally. The cases of CAD are increasing due to the rising aging population, sedentary lifestyle, and other factors. The rising cases of CAD require interventional procedures such as angioplasty as a treatment, which require guidewires, thereby propelling the market.
Thus, rising cases of coronary artery disease require medical interventions, thereby creating guidewires market demand.
Integration of Artificial Intelligence-Powered Sensors is Driving the Guidewires Market Growth
Guidewires are essential for navigating the catheter through complex vascular systems and are pivotal in clinical procedures such as thrombectomy, angioplasty, and others. They typically face resistance while navigating through complex clot formation in these procedures. Integration of artificial intelligence (AI) powered sensors in guidewires detect the characteristics of the clot such as their size, composition, and locations in real-time. It enhances the performance of guidewires by reducing resistance in the navigation of catheters.
- In 2021, Sensome developed the AI-powered, Clotlid smart guidewire. It integrates Sensome’s AI-powered tissue sensor that enables the guidewire to provide physicians with critical information on the clot. It enhances the performance of guidewire, by addressing the detection of clots in real-time. It also reduces resistance in clinical procedures such as thrombectomy, clot removal, and others, thereby boosting the market.
Thus, the development of this smart guidewire is driving the market by providing real-time analysis of clot formation.
Key Restraints :
Stringent Regulatory Approvals are constraining the Guidewire Market Growth
Guidewire manufacturers must adhere to regulatory standards for safety, performance, and biocompatibility. Authorities such as the United States Food and Drug Administration, and the European Medicines Agency (EMA) impose rigorous testing and documentation requirements for guidewires before they are approved for clinical use. These regulations ensure that the guidewires do not cause harm, function effectively in complex procedures and meet the required quality standards. The approval process involves multiple stages, including preclinical trials, testing, biocompatibility evaluations, and clinical trials, which are time-intensive and costly. It typically causes delays due to lengthy evaluations on additional requirements for compliance, particularly for launching innovative products such as AI-integrated guidewires.
Thus, these regulatory approvals cause hurdles in the introduction of new technologies, increasing the cost of product development and limiting the availability of advanced technology, thereby constraining the guidewires market expansion.
Future Opportunities :
Integration of Radiofrequency Technology is expected to create the Guidewires Market Opportunities
Traditional guidewires rely purely on mechanical properties like stiffness, flexibility, and strength to navigate vascular pathways. It typically faces resistance to navigate through heavily calcified occlusion in peripheral vessels. The integration of radiofrequency technology in guidewire allows it to transmit radiofrequency energy to break down and navigate through these occlusions. Further, when this technology comes in contact with the metal of the guidewire, the radiofrequency energy terminates, reducing the surrounding tissue damage.
- In 2024, Baylis Medical Technologies Inc. launched PowerWire® Pro Radiofrequency (RF) Guidewire in the United States. It improves the performance of guidewire by reducing the time needed to navigate through heavily calcified occlusion, further boosting the market.
Thus, this RF-enabled guidewire is expected to create the guidewires market opportunities by reducing the navigation time and improving procedural outcomes.
Guidewires Market Segmental Analysis :
By Product Type:
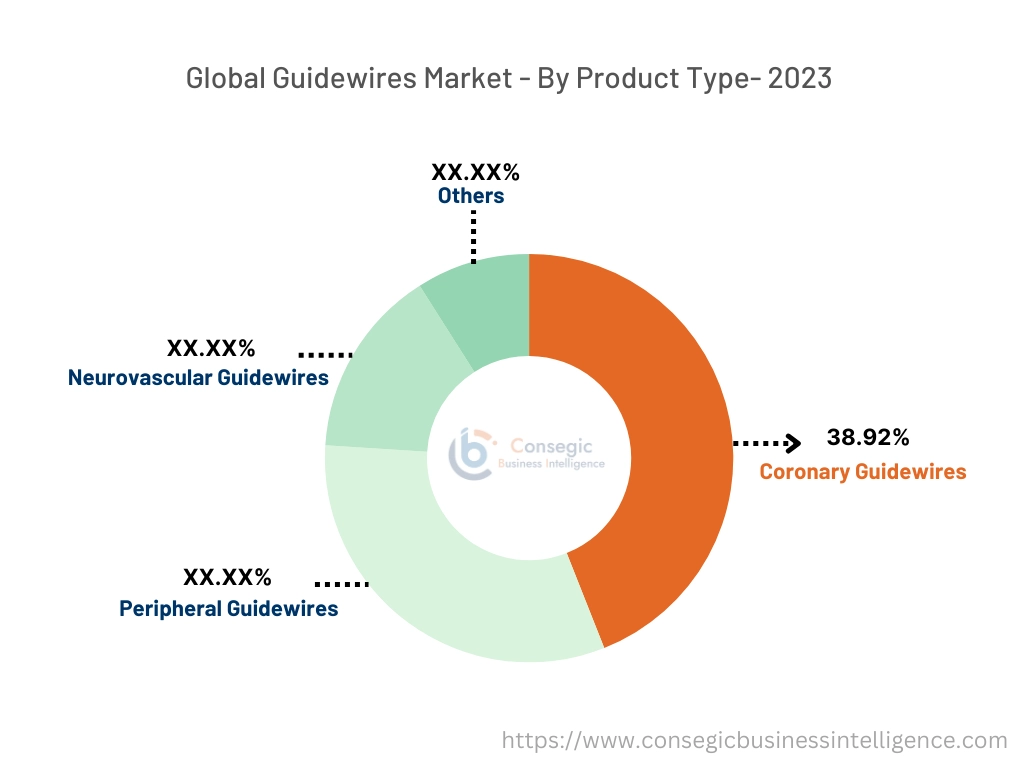
By product type, the market is divided into coronary, peripheral, neurovascular, and others.
Trends in Product Type:
- According to guidewires market trends, coronary guidewires are used in the coronary angioplasty procedure due to high flexibility, and torque control amongst others.
- Use of peripheral guidewires is rising in peripheral artery disease due to greater stiffness and torque control as per the latest trends.
Coronary guidewires accounted for the largest market share of 38.92% in the year 2023.
- Coronary guidewires are specifically designed for use in coronary angioplasty procedures.
- They are used to navigate the catheter through the coronary arteries to reach blocked or narrowed areas. They are typically smaller in diameter around 0.01 to 0.02 inches.
- They have high flexibility, torque control, and usability to allow precise navigation of catheters through the intricate network of coronary vessels.
- They are typically used in crossing chronic total occlusion percutaneous coronary interventions (CTO PCI) procedures. CTO PCI is a minimally invasive procedure used to treat patients with chronic total occlusion or complete blockages of the coronary arteries.
- For instance, in 2022, Teleflex received United States Food and Drug Administration clearance for its coronary guidewires use in CTO PCI procedures. It includes Spectre™ Guidewire, Raider™ Guidewire, Bandit™ Guidewire and others.
- Thus, coronary guidewires are widely used in the market, due to their high flexibility, torque control, and usability.
The peripheral guidewires are expected to grow at the fastest CAGR over the forecast period.
- The emerging use of peripheral guidewires is driven by the rising prevalence of peripheral artery disease due to rising aging populations and lifestyle factors such as diabetes and smoking.
- They are specifically used in interventions targeting the peripheral arteries, such as those in the legs, arms, and renal areas.
- These guidewires are designed to navigate catheters through larger and less tortuous vessels compared to the coronary guidewire, making them ideal for treating peripheral artery disease and other vascular conditions.
- Their robust design with greater stiffness and torque control, helps in crossing calcified occlusions.
- Recent developments include the integration of hydrophilic coatings and advanced materials for enhanced flexibility and smoother navigation.
- Thus, the use of peripheral guidewires is rising due to greater stiffness and flexibility helps in better navigation of catheters as per the latest trends.
By Material:
By material, the market is divided into stainless steel, nitinol, and others.
Trends in Material:
- According to guidewires market trends, stainless steel is widely used in guidewires due to its functionality, reliability, and durability.
- The use of nitinol in guidewires is increasing due to its flexibility and super elasticity as per the latest trends.
Stainless steel accounted for the largest market share in the year 2023.
- Stainless steel is widely due to its mechanical properties, biocompatibility, and corrosion resistance.
- These attributes make it an ideal choice for guidewire, particularly in vascular procedures. In this, guidewire navigates complex and sensitive anatomical structures.
- The use of stainless steel contributes significantly to the functionality, reliability, and durability of guidewire in clinical settings.
- In 2022, Cardio Flow, Inc., announced United States Food and Drug(FDA) clearance for its freedomflow® peripheral guidewire. It is a stainless steel guidewire with a fixed distal spring coil. It is developed to provide exceptional support for diagnostic and therapeutic devices such as C-arm machines, X-ray equipment, and others.
- Thus, stain steel is the most widely used material in the guidewire due to its ability to navigate through complex anatomical structures.
Nitinol is expected to grow at the fastest CAGR over the forecast period.
- The use of nitinol in the production of guidewire is rising due to its super elasticity, biocompatibility, and flexibility.
- Its super elasticity nature allows it to bend and return to its original shape, navigating properly through complex vascular pathways.
- Unlike stainless steel, nitinol guidewire exhibits greater flexibility and kin-resistance, enhancing their performance in delicate procedures.
- Additionally, nitinol’s flexibility enables the guidewire to retain its predefined configuration, ensuring consistent performance even after multiple uses.
- This makes it a preferred choice in interventions such as coronary angioplasty and neurovascular procedures.
- Hence, the use of nitinol in guidewire is rising due to its consistent performance and super elasticity as per the latest trends.
Regional Analysis:
The regional segment includes North America, Europe, Asia Pacific, the Middle East and Africa, and Latin America.
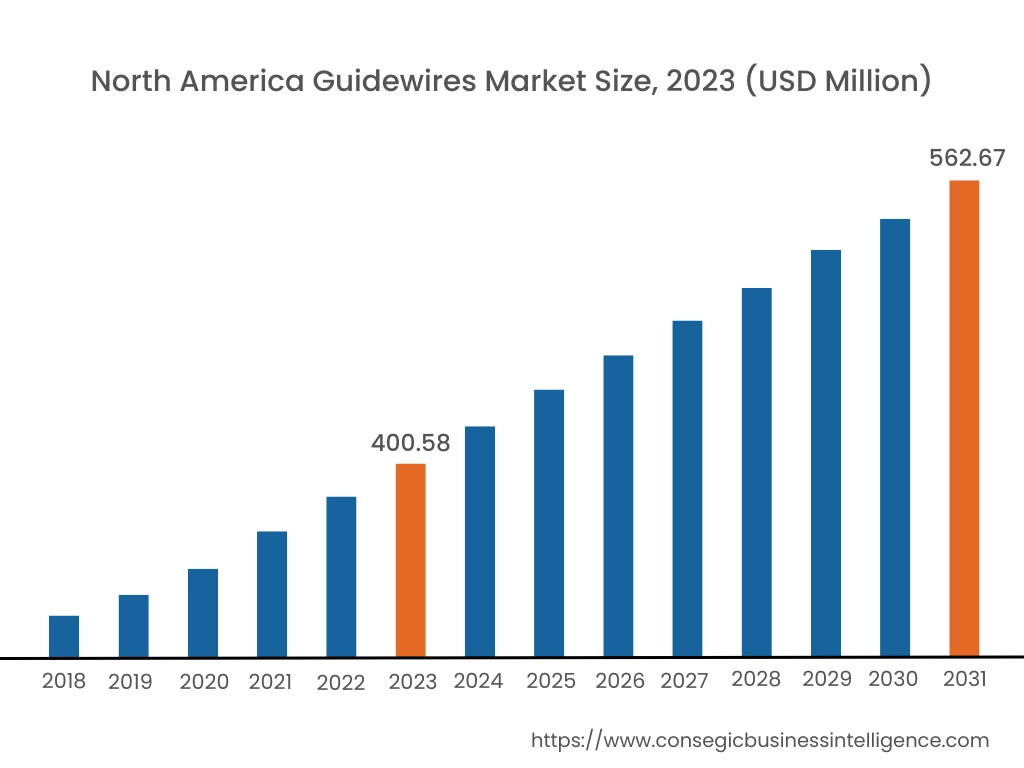
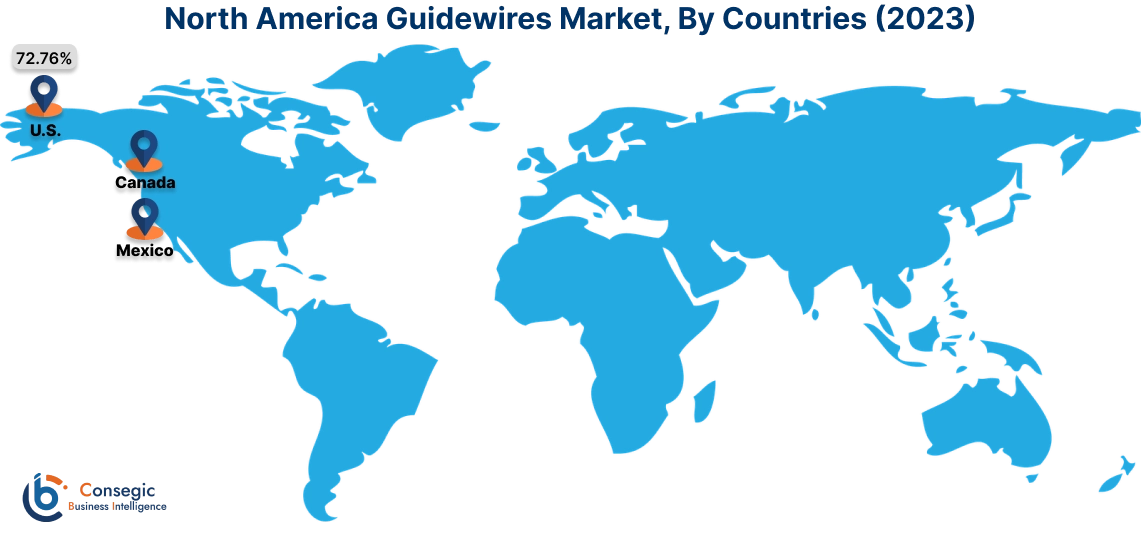
In 2023, North America accounted for the highest market share at 38.25% and was valued at USD 400.58 Million and is expected to reach USD 562.67 Million in 2031. In North America, the U.S. accounted for the highest market share of 72.76% during the base year of 2023. The guidewires market share of the North American region is supported by a rising number of coronary artery diseases, advanced machinery, and infrastructural developments. The population in this region prefers minimally invasive techniques such as angioplasty and thrombectomy where guidewires are necessary, further propelling the market. Additionally, key players such as Philips, Medtronic, Boston Scientific, and others are launching innovative guidewires in turn accelerating the market growth.
- In 2024, Philips introduced a 160cm US Food and Drug Administration (FDA)-approved version of its LumiGuideendovascular navigation wire. This enhanced LumiGuide guidewire utilizes the company’s Fiber Optic Real Shape (FORS) technology. It was used for the first time on a United States patient during a complex aortic aneurysm repair operation. It enables US clinicians to visualize a broader range of catheters using this guidewire, promoting the market.
Thus, advancement in minimally invasive techniques and well-established healthcare infrastructure is driving the market in this region.
Asia-Pacific is expected to witness the fastest CAGR over the forecast period of 5.1% during 2024-2031. According to the market analysis, the Asia-Pacific guidewires market growth is increasing, driven by the increasing prevalence of cardiovascular diseases, early diagnosis, and rising healthcare spending. Moreover, the growing geriatric population and rising adoption of minimally invasive techniques such as thrombectomy and angioplasty are progressing in the region. Countries like Japan, China, and South Korea are key contributors in the market expanding health insurance coverage and the development of new healthcare facilities. Technological advancements, increased consumer demand, and evolving regulatory frameworks are resulting in guidewires market expansion in the region.
Thus, the guidewires market share of Asia-Pacific is expanding supported by a rising geriatric population, favorable regulatory frameworks, and increased healthcare spending.
According to guidewires market analysis, the market in the Europe region is characterized by well-established healthcare systems, increased government spending, and a focus on minimally invasive techniques. With a rising elderly population and growing rates of chronic diseases, the demand for minimally invasive techniques that require guidewires has significantly increased. The market benefits from advanced healthcare infrastructure, particularly in Western Europe due to advanced healthcare facilities and skilled healthcare professionals. Increasing cases of peripheral artery disease, stroke, valve replacement procedures, and others are increasing in the region, further propelling the market. Countries such as Germany, France, and the United Kingdom are key contributors to the market due to the presence of global players such as Medtronic, Boston Scientific, Abbott, and others.
As per guidewires market analysis, the market is growing rapidly in the Middle East and Africa region, especially in Gulf Cooperation Council (GCC) countries. It is driven by the rising prevalence of cardiovascular diseases and increasing government investment in healthcare infrastructure. Countries such as Dubai, UAE, and South Africa are establishing advanced healthcare facilities, which are providing minimally invasive treatment further accelerating the market. The market's growth is bolstered by factors such as rising incidences of lifestyle-related diseases, increasing healthcare expenditure, and government initiatives to expand healthcare infrastructure. Moreover, the UAE's position as a hub for medical tourism further drives demand for minimally invasive treatments where guidewires are necessary, expanding the market in this region.
In Latin America, minimally invasive treatments are growing steadily, fueled by the rising incidence of coronary artery disease, improving healthcare infrastructure, and increasing government support. Countries like Brazil, Mexico, and Argentina are major contributors to the market, supported by skilled healthcare professionals and advanced healthcare facilities. Further, governments in these countries are providing robust healthcare policies and insurance coverage for patients availing treatments in healthcare facilities. Key factors driving growth include a higher prevalence of peripheral artery diseases, stroke, and others due to lifestyle changes and the aging population. However, widespread access to minimally invasive treatments is limited due to economic disparity, and lack of advanced healthcare infrastructure in some parts of the region hindering the market as per analysis.
Top Key Players & Market Share Insights:
The global guidewires market is highly competitive with major players providing products to the national and international markets. Key players are adopting several strategies in research and development (R&D) and product innovation to hold a strong position in the market. Key players in the guidewires industry include-
- Boston Scientific Corporation (United States)
- Abbott (United States)
- Braun (Germany)
- Biotronik (Germany)
- Advin Health Care (India)
Recent Industry Developments :
Business Expansion:
- In 2024, Sensome signed an exclusive commercial distribution agreement for clot-sensing guidewire in Japan with Cosmotec. This clot-sensing guidewire integrates electrical sensors and instantly identifies clot composition and clot length in real-time. It will enhance the performance of guidewires, thereby promoting the market.
Product Launches:
- In 2024, Medtroniclaunched the Steerant Aortic Guidewire, tailored to facilitate catheter placement and exchange during diagnostic or interventional procedures in the aorta. It is 15 cm soft and its polytetrafluoroethylene (PTFE) coating provides smooth device delivery. It provides balanced control in complex anatomy, further fueling the market growth
- In 2024, Xenter Inc. launched its dual sensor investigational guidewire for transcatheter aortic valve replacement (TAVR) procedures. It is designed to operate seamlessly within a proprietary wireless ecosystem in the cardiac catheterization lab. It will lead to real-time data and analysis more accessible, creating innovation in the market.
Guidewires Market Report Insights :
| Report Attributes | Report Details |
| Study Timeline | 2018-2031 |
| Market Size in 2031 | USD 1,468.33 Million |
| CAGR (2024-2031) | 4.4% |
| By Product Type |
|
| By Material |
|
| By Region |
|
| Key Players |
|
| North America | U.S. Canada Mexico |
| Europe | U.K. Germany France Spain Italy Russia Benelux Rest of Europe |
| APAC | China South Korea Japan India Australia ASEAN Rest of Asia-Pacific |
| Middle East and Africa | GCC Turkey South Africa Rest of MEA |
| LATAM | Brazil Argentina Chile Rest of LATAM |
| Report Coverage |
|
Key Questions Answered in the Report
How big is the Guidewires Market? +
Guidewires market size is estimated to reach over USD 1,468.33 Million by 2031 from a value of USD 1,047.28 Million in 2023, growing at a CAGR of 4.4% from 2024 to 2031.
What specific segmentation details are covered in the Guidewires Market report? +
The Guidewires Market report includes specific segmentation details for product type and material.
Which is the fastest growing region in the Guidewires Market? +
Asia Pacific is the fastest-growing region in the Guidewires Market.
Who are the major players in the Guidewires Market? +
The key participants in the Guidewires market are Boston Scientific Corporation (United States), Abbott (United States), Medtronic (United States), Stryker (United States), Cardinal Health (United States), Terumo (Japan), Cordis (United States), B. Braun (Germany), Biotronik (Germany) Advin Health Care (India).