- Summary
- Table Of Content
- Methodology
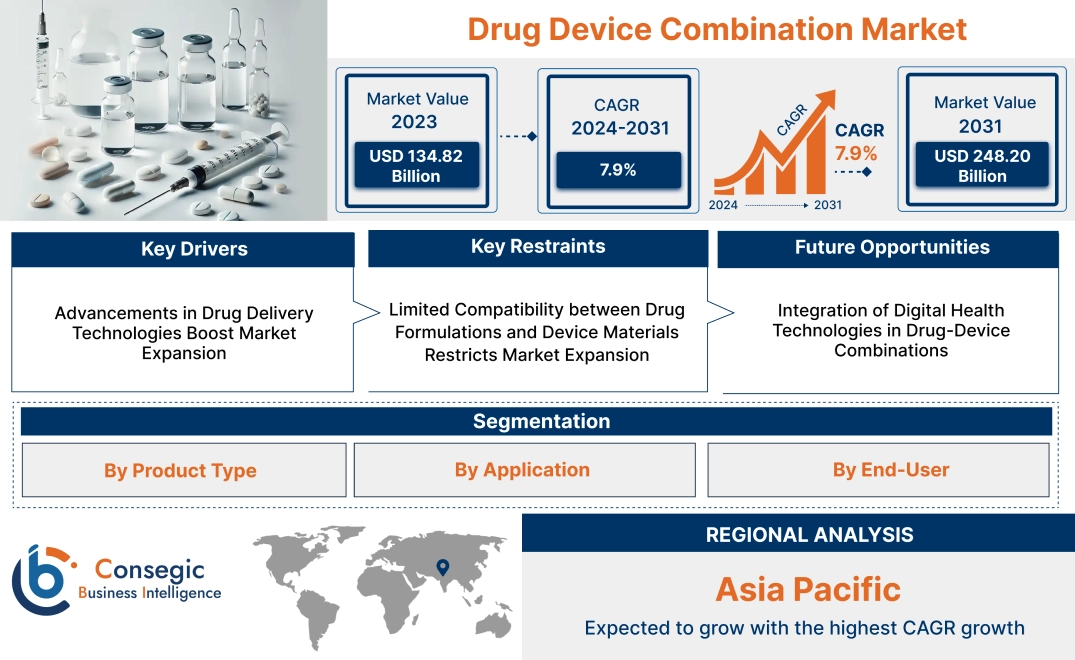
Drug Device Combination Market Size:
Drug Device Combination Market size is estimated to reach over USD 248.20 Billion by 2031 from a value of USD 134.82 Billion in 2023 and is projected to grow by USD 143.11 Billion in 2024, growing at a CAGR of 7.9% from 2024 to 2031.
Drug Device Combination Market Scope & Overview:
The drug-device combination market analysis focuses on products that integrate medical devices with pharmaceutical agents to deliver targeted therapies and enhance treatment outcomes. These combination products include drug-eluting stents, inhalers, pre-filled syringes, and infusion pumps, which combine the functionalities of a drug and a device into a single, innovative solution. Key characteristics include precise drug delivery, improved therapeutic efficacy, and reduced systemic side effects. The benefits of drug-device combinations include enhanced patient compliance, reduced hospitalization, and minimized procedural risks. Applications span various therapeutic areas such as cardiovascular diseases, diabetes management, respiratory disorders, and oncology. End-users include hospitals, ambulatory surgical centers, and specialty clinics, driven by the increasing prevalence of chronic diseases, advancements in combination technologies, and rising drug device combination market demand for minimally invasive treatment options.
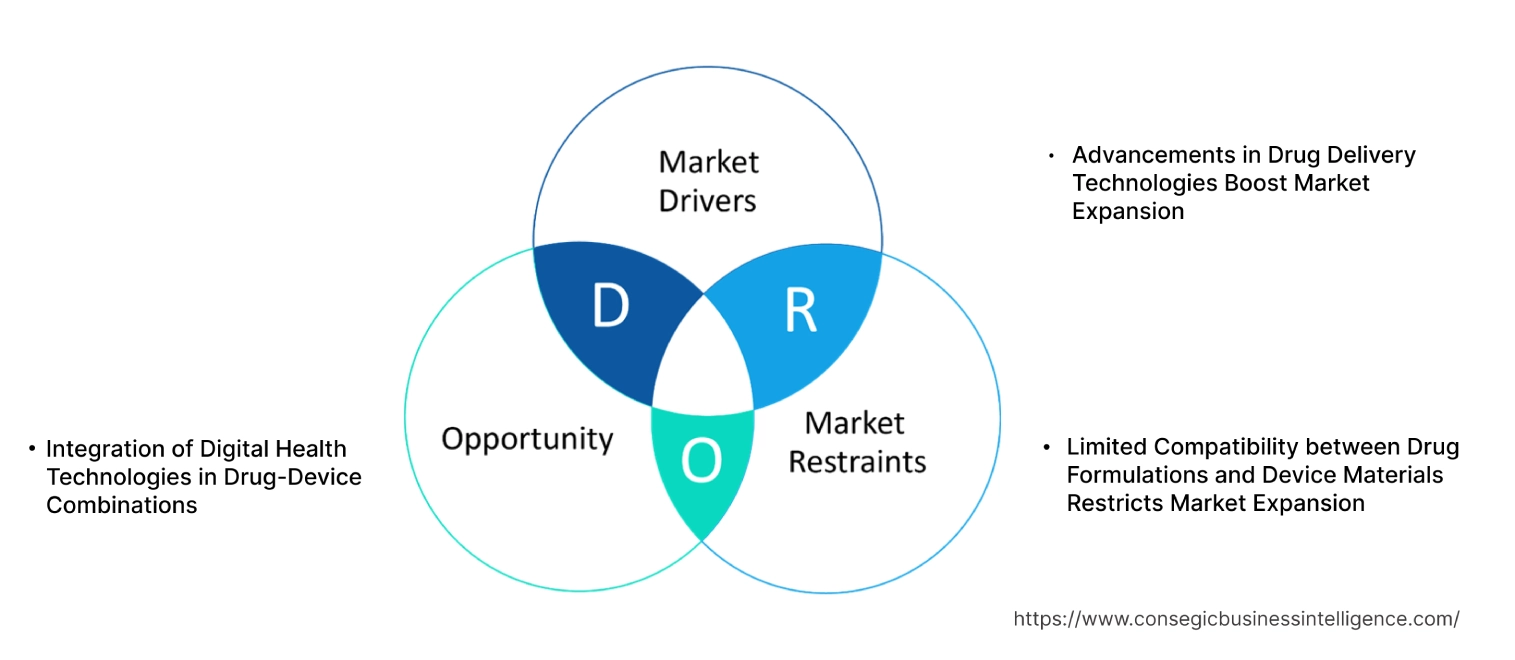
Drug Device Combination MarketDynamics - (DRO) :
Key Drivers:
Advancements in Drug Delivery Technologies Boost Market Expansion
Favorable Regulatory Framework for Sustainable Packaging is Fuelling the Market Growth
The rapid advancements in drug delivery technologies are significantly driving the drug device combination market growth. Innovations such as auto-injectors, wearable injectors, and inhalable drug delivery systems are transforming how medications are administered, providing greater accuracy, convenience, and efficacy. These technologies ensure controlled and precise drug dosages, improving therapeutic outcomes and reducing side effects.
For example, wearable injectors allow continuous subcutaneous drug delivery over an extended period, which is particularly beneficial for biologics used in chronic conditions like rheumatoid arthritis and cancer. Additionally, advancements in microneedle technology have enabled minimally invasive drug administration, enhancing patient comfort and compliance. These developments cater to the increasing demand for personalized medicine, where specific drug delivery mechanisms are designed to suit individual patient needs, further driving the drug device combination market expansion.
Key Restraints :
Limited Compatibility between Drug Formulations and Device Materials Restricts Market Expansion
Compatibility issues between drug formulations and the materials used in delivery devices are a significant challenge for the drug-device combination market analysis. Certain drugs, particularly biologics and protein-based therapies, can interact negatively with device materials, leading to reduced drug stability, contamination, or altered therapeutic efficacy. For instance, biologics may adhere to or degrade within syringes or auto-injectors made of conventional polymers, requiring specialized coatings or material modifications. These compatibility concerns necessitate extensive testing and development of new device materials that can safely store and deliver sensitive drugs. This process increases costs, lengthens development timelines, and poses challenges for integrating novel drug formulations into existing device platforms. Overcoming these issues is particularly critical in applications like oncology and gene therapy, where maintaining drug integrity is vital for patient outcomes.
Future Opportunities :
Integration of Digital Health Technologies in Drug-Device Combinations
The integration of digital health technologies into drug-device combinations is revolutionizing the market by enhancing treatment monitoring, patient adherence, and overall therapeutic efficacy. Connected devices, such as smart inhalers and Bluetooth-enabled auto-injectors, allow patients and healthcare providers to track real-time usage data. This integration enables better monitoring of adherence rates and ensures accurate administration of prescribed doses. Additionally, smart devices can alert patients about missed doses or improper usage, improving compliance and treatment outcomes.
These devices often link to mobile apps or cloud-based platforms, allowing remote monitoring by healthcare providers and enabling personalized treatment adjustments. For example, in asthma management, connected inhalers provide detailed insights into a patient's usage patterns and environmental triggers, leading to more effective treatment plans. The growing adoption of such digital health solutions aligns with the broader trend toward value-based healthcare, creating significant drug device combination market opportunities for drug-device combination manufacturers to innovate and meet evolving patient and provider needs.
Drug Device Combination Market Segmental Analysis :
By Product Type:
Based on product type, the market is segmented into infusion pumps (Volumetric, Disposables, Syringes, Ambulatory, Implantable, and Insulin), orthopedic combination products (Bone Graft Implants and Antibiotic Bone Cement), photodynamic therapy devices, transdermal patches, drug-eluting stents (Coronary Stents and Peripheral Vascular Stents), wound care products, inhalers (Dry Powder, Nebulizers, and Metered Dose), antimicrobial catheters (Urological and Cardiovascular), and others.
The infusion pumps segment accounted for the largest revenue share in 2023.
- Infusion pumps, including volumetric, disposable, syringe, ambulatory, implantable, and insulin pumps, are critical in delivering precise medication dosages in various healthcare settings.
- Insulin pumps dominate the sub-segment due to the rising prevalence of diabetes and advancements in pump technologies, including automated and smartphone-integrated systems.
- Ambulatory pumps are gaining traction for their portability and use in homecare settings, especially in pain management and oncology treatments.
- The increasing adoption of infusion pumps in chronic disease management, supported by technological advancements, drives the dominance of this segment.
- Hence, infusion pumps lead analysis of the drug device combination market trends, driven by their critical role in chronic disease management, especially diabetes, and the growing advancement for portable and advanced devices.
The transdermal patches segment is anticipated to register the fastest CAGR during the forecast period.
- Transdermal patches provide a non-invasive drug delivery method, offering controlled release of medication over time.
- They are extensively used for pain management, hormonal therapy, and smoking cessation.
- The growing focus on patient compliance and advancements in transdermal patch technologies, including microneedle patches, are driving the segment's rapid development.
- The shift towards minimally invasive drug delivery solutions and increased adoption in managing chronic conditions like hypertension and neurological disorders further support the market surge.
- Therefore, transdermal patches are expected to grow rapidly, supported by their non-invasive delivery method and the increasing focus on patient compliance and convenience surges in market trends.
By Application:
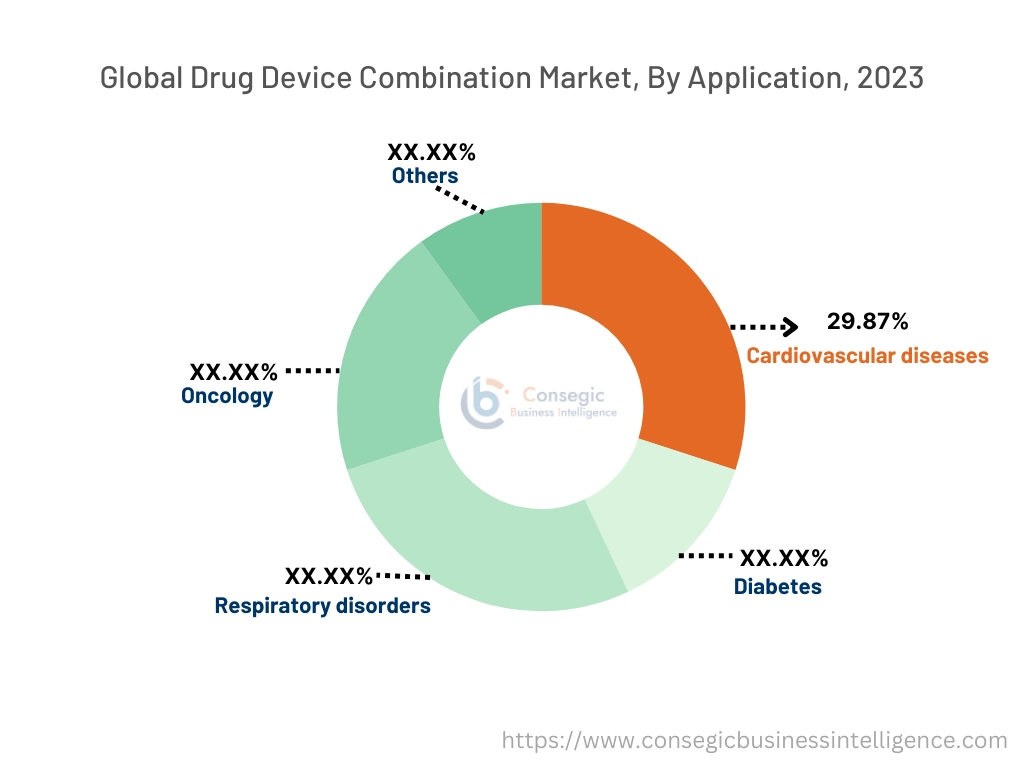
Based on application, the market is segmented into cardiovascular diseases, diabetes, respiratory disorders, oncology, and others.
The cardiovascular diseases segment accounted for the largest revenue share of 29.87% in 2023.
- Cardiovascular applications dominate the market due to the extensive use of drug-device combination products like drug-eluting stents, antimicrobial catheters, and implantable infusion pumps.
- Drug-eluting stents, particularly coronary stents, play a vital role in managing atherosclerosis and improving patient outcomes.
- The increasing prevalence of cardiovascular diseases, driven by aging populations and sedentary lifestyles, fuels drug device combination market demand for these products.
- Additionally, advancements in stent technologies, including biodegradable coatings, enhance their adoption in interventional cardiology.
- Cardiovascular diseases lead the drug device combination market expansion, driven by the high prevalence trends of heart conditions and advancements in stent and catheter technologies.
The oncology segment is anticipated to register the fastest CAGR during the forecast period.
- The use of drug-device combinations in oncology, such as infusion pumps, photodynamic therapy devices, and transdermal patches, is expanding due to their role in delivering targeted and sustained drug therapies.
- The growing focus on precision medicine and the increasing incidence of cancer globally are driving this segment’s rapid development.
- Technological advancements in devices that enable precise dosing and minimize systemic side effects are further supporting adoption in oncology treatments.
- Therefore, oncology is expected to grow rapidly, driven by advancements in trends in targeted drug delivery systems and the increasing focus on precision medicine in cancer care.
By End-User:
Based on end-user, the market is segmented into hospitals & clinics, ambulatory surgical centers (ASCs), homecare settings, and others.
The hospitals & clinics segment accounted for the largest revenue share in 2023.
- Hospitals and clinics are the primary users of drug-device combination products creating new drug device combination market opportunities, particularly for critical care applications such as cardiovascular interventions, oncology treatments, and chronic disease management.
- The extensive availability of advanced medical devices and expertise in hospitals supports the segment’s dominance.
- Additionally, the increasing number of hospitalizations for chronic diseases and the adoption of minimally invasive treatment approaches are boosting the surge for combination products in these settings.
- Hence, hospitals & clinics dominate the market trends, driven by their critical role in managing chronic and acute conditions with advanced drug-device combination products.
The homecare settings segment is anticipated to register the fastest CAGR during the forecast period.
- Homecare settings are witnessing increased adoption of drug-device combinations like insulin pumps, ambulatory infusion pumps, and transdermal patches, driven by the growing demand for patient-centric care and convenience.
- The rising prevalence of chronic diseases such as diabetes and respiratory disorders is fueling the shift towards homecare solutions.
- Additionally, advancements in portable and user-friendly devices are enhancing the feasibility of home-based treatments, further propelling this segment's drug device combination market growth.
- Hence, homecare settings trends are expected to grow rapidly, supported by the increasing shift towards patient-centric care and the rising adoption of portable and user-friendly combination devices.
Regional Analysis:
The regional segment includes North America, Europe, Asia Pacific, the Middle East and Africa, and Latin America.
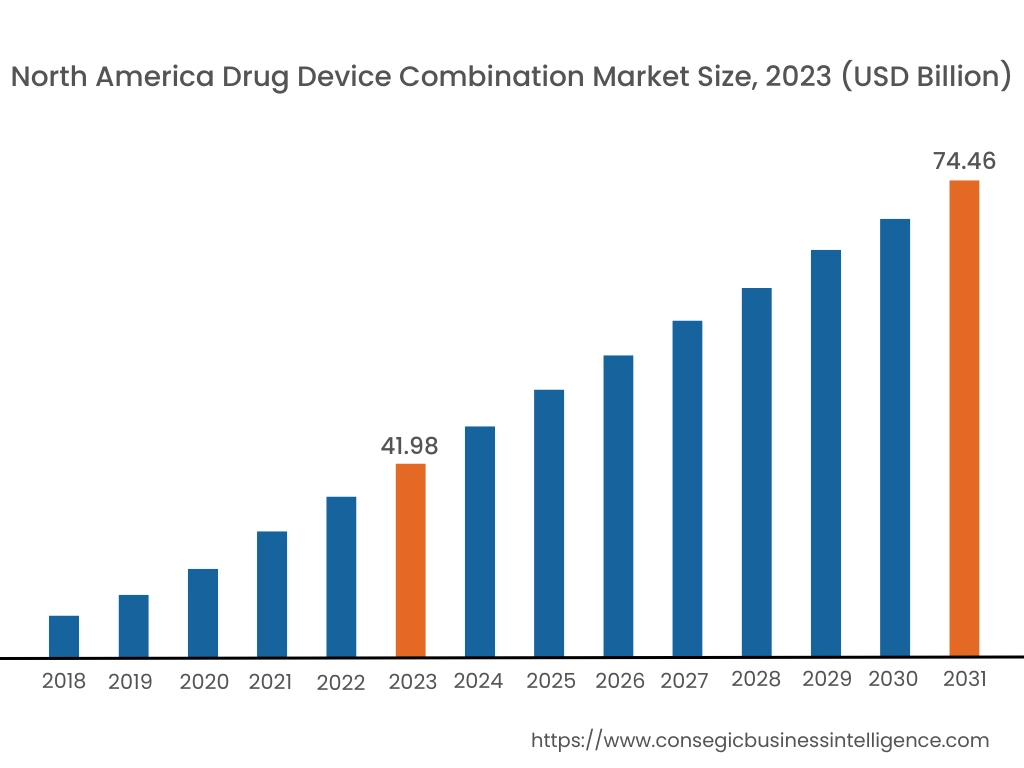
In 2023, North America was valued at USD 41.98 Billion and is expected to reach USD 74.46 Billion in 2031. In North America, the U.S. accounted for the highest drug device combination market share of 72.34% during the base year of 2023. North America holds the largest drug device combination market share, driven by strong demand for advanced drug delivery systems and well-established healthcare infrastructure. The U.S. leads the region due to the increasing prevalence of chronic diseases such as diabetes and cardiovascular conditions, which drive the development of combination products like insulin pens, inhalers, and drug-eluting stents. The presence of key market players, extensive R&D investments, and favorable reimbursement policies further boost market advancement. Canada is also contributing through its focus on innovative medical devices and increased adoption of combination therapies, although stringent regulatory frameworks and high development costs pose challenges.
In Asia Pacific, the market is experiencing the fastest growth with a CAGR of 8.2% over the forecast period. Asia-Pacific is the fastest-growing region in the drug device combination market trends, driven by rapid healthcare advancements, increasing prevalence of chronic diseases, and growing demand for innovative drug delivery systems in China, Japan, and India. China is leading the region due to its expanding pharmaceutical and medical device sectors, with a focus on producing affordable combination products for chronic disease management. Japan’s aging population and advanced healthcare system are fueling a surge for implantable devices and drug-eluting stents. India’s growing diabetic and respiratory patient population is driving the adoption of insulin pens and inhalers. However, according to the regional analysis it faces challenges related to inconsistent regulatory frameworks and limited access to cutting-edge technologies in certain areas.
Europe is a significant market for drug devices, supported by the growing demand for minimally invasive treatments and advanced drug delivery technologies. The analysis depicts countries like Germany, the UK, and France leading the market, with strong adoption of products like prefilled syringes, transdermal patches, and implantable drug delivery devices. Germany’s advanced healthcare system and focus on innovation in combination therapies, particularly in oncology and cardiovascular care, drive growth. The UK is witnessing increased use of self-administration devices such as auto-injectors, reflecting a shift toward patient-centric care. However, stringent regulatory requirements under the European Medical Device Regulation (MDR) pose challenges for manufacturers in the region.
The analysis portrays the Middle East & Africa region as experiencing moderate growth in the market, with increasing investments in healthcare infrastructure and a rising prevalence of chronic conditions such as diabetes and asthma. The UAE and Saudi Arabia are the key markets, focusing on the adoption of advanced drug delivery systems like inhalers and insulin pens. In Africa, South Africa is witnessing growth in combination products for respiratory and diabetic care, driven by increasing awareness and improving healthcare access. However, limited local manufacturing capabilities and reliance on imported products hinder broader market growth in the region.
Latin America is an emerging market for drug devices, with Brazil and Mexico leading the region. Brazil’s growing diabetic population and government initiatives to improve healthcare services are driving a surge for insulin pens and prefilled syringes. Mexico’s expanding medical device manufacturing sector and increasing focus on chronic disease management support market growth. The region is also seeing growing interest in implantable drug delivery devices for oncology and cardiovascular care. However, economic instability and limited R&D capabilities for advanced combination products present challenges for market surge.
Top Key Players & Market Share Insights:
The Drug Device Combination market is highly competitive with major players providing products and services to the national and international markets. Key players are adopting several strategies in research and development (R&D), product innovation, and end-user launches to hold a strong position in the global Drug Device Combination market. Key players in the Drug Device Combination industry include -
- Abbott Laboratories (United States)
- Medtronic plc (Ireland)
- Becton, Dickinson, and Company (United States)
- Novartis International AG (Switzerland)
- Smith & Nephew plc (United Kingdom)
- Boston Scientific Corporation (United States)
- Stryker Corporation (United States)
- Terumo Corporation (Japan)
- Mylan N.V. (United States)
- Allergan plc (Ireland)
Recent Industry Developments :
Product Launches:
- In August 2024, Dexcom launched its over-the-counter continuous glucose monitor, Stelo, in the United States, marking a significant industry milestone. Approved by U.S. regulators in March for adults aged 18 and older who do not use insulin, Stelo is the first OTC continuous glucose monitor available in the market. Priced at $99 for a pack of two sensors or $89 monthly via subscription, this innovation aims to improve accessibility to glucose monitoring solutions.
Approvals:
- In April 2024, Abbott secured FDA approval for its TriClip transcatheter edge-to-edge repair (TEER) system, enhancing its portfolio of structural heart therapies. The device addresses tricuspid regurgitation, marking a significant advancement in cardiac care solutions.
Drug Device Combination Market Report Insights :
| Report Attributes | Report Details |
| Study Timeline | 2018-2031 |
| Market Size in 2031 | USD 192,200.11 Million |
| CAGR (2024-2031) | 12.2% |
| By Product Type |
|
| By Application |
|
| By End-User |
|
| By Region |
|
| Key Players |
|
| North America | U.S. Canada Mexico |
| Europe | U.K. Germany France Spain Italy Russia Benelux Rest of Europe |
| APAC | China South Korea Japan India Australia ASEAN Rest of Asia-Pacific |
| Middle East and Africa | GCC Turkey South Africa Rest of MEA |
| LATAM | Brazil Argentina Chile Rest of LATAM |
| Report Coverage |
|
Key Questions Answered in the Report
What is the size of the drug-device combination market? +
Drug Device Combination Market size is estimated to reach over USD 248.20 Billion by 2031 from a value of USD 134.82 Billion in 2023 and is projected to grow by USD 143.11 Billion in 2024, growing at a CAGR of 7.9% from 2024 to 2031.
What factors are driving the market growth? +
Increasing prevalence of chronic diseases, advancements in minimally invasive devices, and rising demand for personalized medicine.
Which regions dominate the drug-device combination market? +
North America leads the market, followed by Europe, due to advanced healthcare infrastructure and high R&D investments.
What are some examples of drug-device combinations? +
Drug-eluting stents, inhalers, transdermal patches, and antimicrobial catheters.
What are the key challenges in the market? +
Regulatory complexities, high development costs, and ensuring compatibility between drugs and devices.