- Summary
- Table Of Content
- Methodology
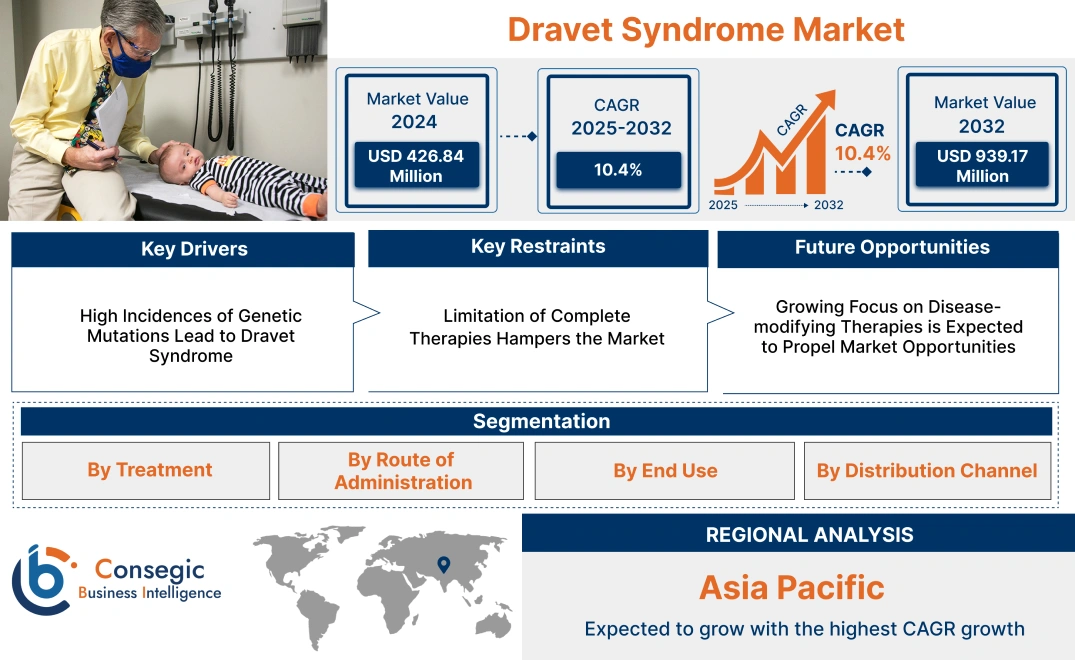
Dravet Syndrome Market Size:
Consegic Business Intelligence analyzes that the Dravet Syndrome market size is growing with a CAGR of 10.4% during the forecast period (2025-2032). The market accounted for USD 426.84 Million in 2024 and USD 463.45 Million in 2025, and the market is projected to be valued at USD 939.17 Million by 2032.
Dravet Syndrome Market Scope & Overview:
Dravet syndrome is an intractable developmental and epileptic encephalopathy that begins in infancy and proceeds with accumulating morbidity that significantly impacts individuals throughout their lifetime. It is characterized by frequent, prolonged, and refractory seizures that usually begin within the first year of life. This syndrome is classified as a developmental and epileptic encephalopathy due to developmental delays and cognitive impairment, in addition to seizure activity. The majority of patients with this syndrome carry a mutation in the sodium channel gene SCN1A that affects the functioning of sodium channels. As per the analysis, the group of comorbidities addressed in This syndrome are intellectual disability, movement and balance issues, defects, sleep abnormalities, disruptions of the autonomic nervous system, and photosensitivity, among others. The primary goal of seizure treatment is to find the best combination of medications for a child's long-term condition.
Dravet Syndrome Market Insights:
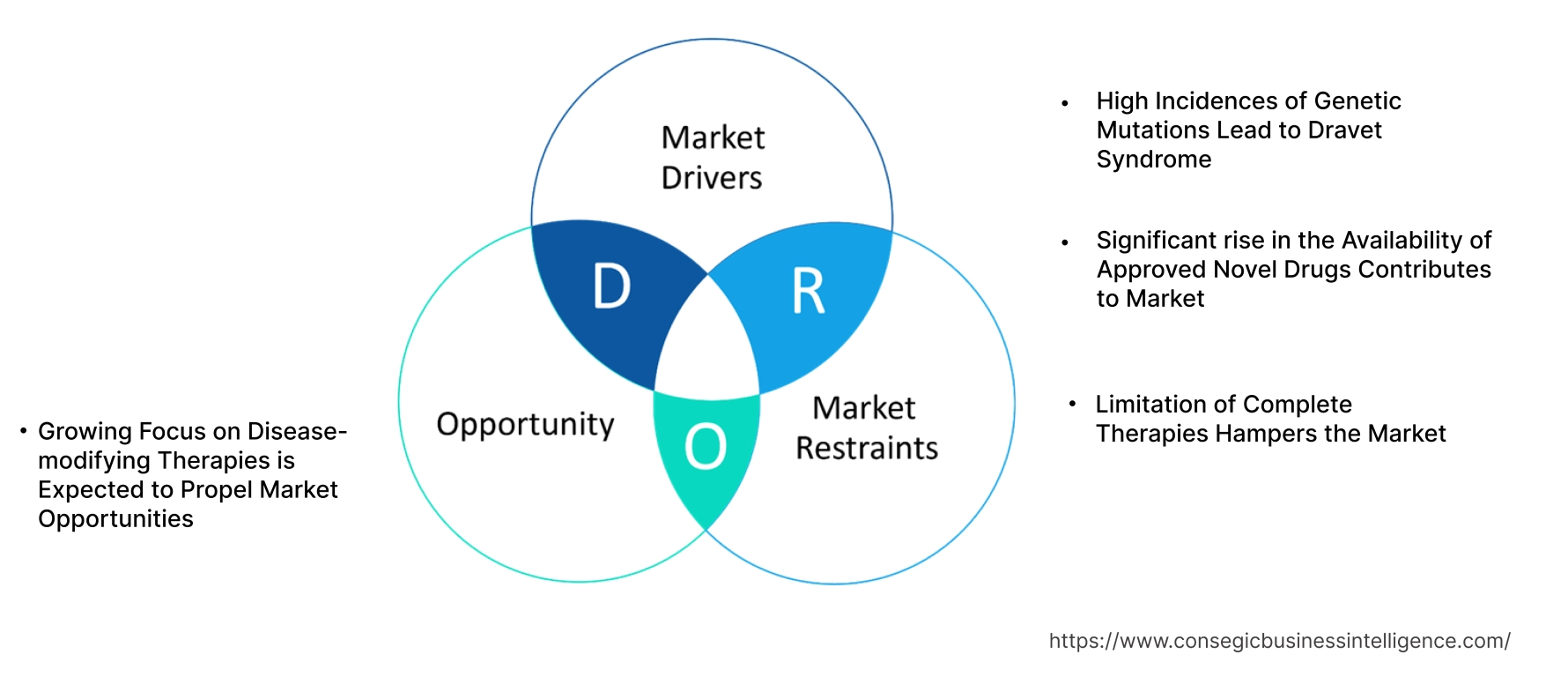
Key Drivers:
High Incidences of Genetic Mutations Lead to Dravet Syndrome
Genetic mutation is the permanent alteration in the DNA sequence of an organism. The syndrome is a rare progressive genetic epilepsy caused by a mutation in the sodium channel gene SCN1A. This syndrome is an autosomal dominant disorder that leads to developmental delays and cognitive impairment, in addition to severe seizure activity. The high incidence of this syndrome due to genetic mutation is the potential factor driving the demand for effective management of the syndrome.
- For instance, the National Organization for Rare Disorders in July 2020, in Dravet syndrome, a severe form of epilepsy, over 80% of patients have been found to have a genetic mutation specifically in the SCN1A gene.
Thus, the rising incidences of genetic mutations lead to the development of this syndrome which is driving the market.
Significant rise in the Availability of Approved Novel Drugs Contributes to Market
The potential rise in the availability of advanced drugs approved specifically for the management of this syndrome is a substantial factor fuelling the rise of the market. Traditionally, first, second, and third-line medications including clobazam and valproic acid listed for the management of seizures are available as the treatment recommendation. Apart from these primary medications, commendable efforts made by the biopharmaceutical and pharmaceutical companies are influencing regulatory agencies to create progress in the accessibility of emerging drugs.
- For instance, in June 2020, the U.S. Food and Drug Administration announced the approval of Fintepla (fenfluramine), a schedule IV controlled substance, for the treatment of seizures associated with Dravet syndrome in patients age 2 and older. Further, in July 2022, the US Food and Drug Administration authorized the use of stiripentol (DIACOMIT) for patients with this syndrome aged 6 months and older and taking clobazam.
Overall, the progress in the availability of advanced drugs for this syndrome is surging the market.
Key Restraints :
Limitation of Complete Therapies Hampers the Market
Early diagnosis and prompt treatment are critical to prevent high morbidity and its overwhelming effect on the quality of life of children. Currently, this syndrome is being managed primarily by antiseizure medications that prevent the recurrence of seizures. However, there is limited approved therapy treatment for this syndrome which particularly modifies the mutated gene. This limits the patients and healthcare providers to prevail effective treatment. The unavailability of specific treatment selectively targeting mutated genes makes it challenging to completely cure the syndrome. Consequently, the lack of a whole disease-modifying treatment for this syndrome is posing a significant threat to the market.
Future Opportunities :
Growing Focus on Disease-modifying Therapies is Expected to Propel Market Opportunities
The demand for more advanced solutions for the treatment of this syndrome has motivated the rise in research and development activities. Disease-modifying therapies (DMTs) are treatments that target the underlying cause of a disease and aim to delay, slow, or reverse its progression. Based on the analysis, of the context of multiple sclerosis (MS), DMTs are designed to modify the immune system's response and prevent it from attacking the myelin sheath that protects nerve cells. Disease-modifying therapies for this syndrome focus on reducing seizure frequency and improving overall patient outcomes. However, the biopharmaceutical and pharmaceutical companies are accelerating their efforts for manufacturing novel medications such as RNA-based medications for the development of gene therapies that cater to the potential to treat this syndrome.
- For instance, in March 2024, Stroke Therapeutic Inc., a biotech firm specializing in tackling the root cause of serious illnesses by increasing protein production with RNA-based drugs revealed that discussions regarding the company's research on the STK-001 about this syndrome will be featured at the American Epilepsy Society's 2023 Annual Meeting. The firm is making progress with STK-001 with the potential of it being the first drug to target the genetic basis of this syndrome.
Thus, consistent demand to come up with innovative solutions is expected to create enormous opportunities in the market.
Dravet Syndrome Market Segmental Analysis :
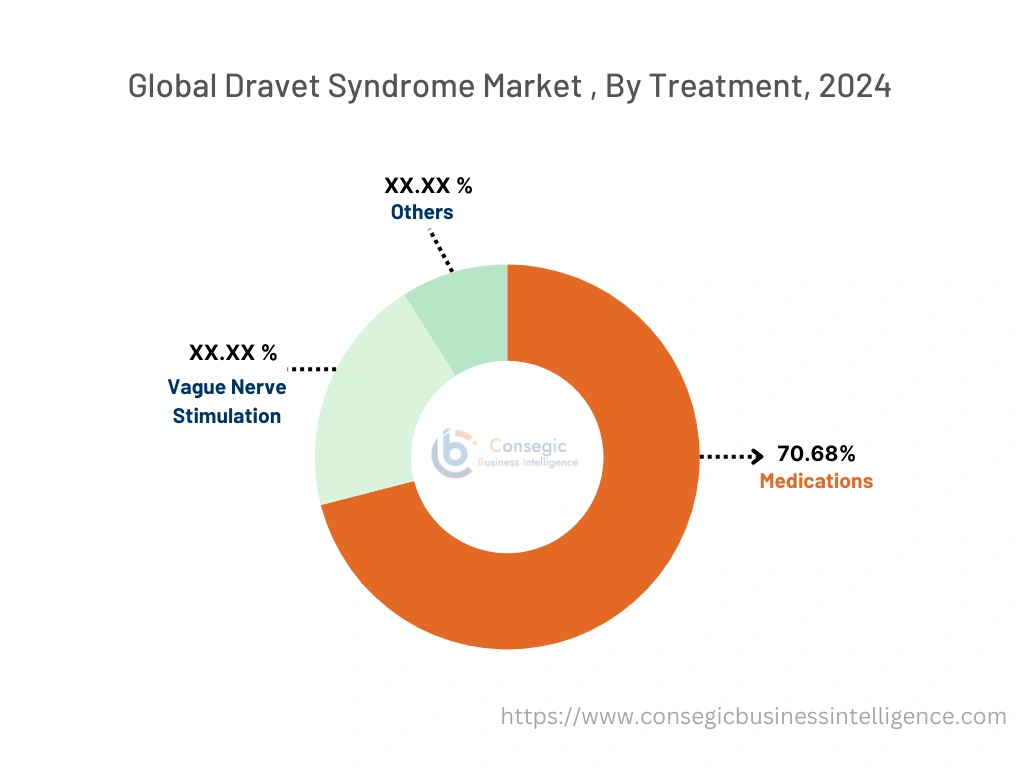
By Treatment:
The treatment is categorized into medications, vague nerve stimulation, and others.
Trends in the Treatment:
- Increase in the use of therapies such as ETX101, and STK-001 for the treatment.
- Increasing regulatory approval and special designation of drugs such as fast track designation, and accelerated approval among others.
In 2024, the medications segment accounted for the highest market share of 70.68% in the Dravet syndrome market and it is also expected to hold the fastest CAGR over the forecast period.
- The medications segment is further segmented into stiripentol, cannabidiol, fenfluramine, clobazam, valproate, and others.
- These medications act by altering electrical activity in neurons by affecting sodium ion channels in the cell membrane.
- They are readily available and are preferred as the initial and escalation therapy for the management of this syndrome.
- These medications work by regulating gene and protein expression and are expected to be widely adopted for comprehensive disease modification.
- Additionally, as per the analysis, the efforts taken by leading companies to promote the availability of advanced medications are contributing to the potential of the segment.
- For instance, in February 2024, Encoded Therapeutics Inc., announced the outline of the global development strategy for its lead gene therapy candidate, ETX101, for the treatment of SCN1A+ syndrome. ETX101 is an AAV9-mediated candidate gene regulation therapy designed to selectively upregulate expression of the SCN1A gene to potentially address the underlying cause of the disease. In addition to this, the company plans to initiate the phase 1/2 clinical trial ENDEAVOR (NCT05419492), a 2-part dose escalation study in the US, to assess the therapy in patients between the ages of 6 months to 3 years with SCN1A+ DS in the first half of 2024.
- As a result, the easy availability and the rising efforts to provide cutting-edge medications are forging the segment trend.
By Route of Administration:
Based on application, the Dravet Syndrome Market has been segmented into Remote Monitoring, Diagnostics and Treatment, Disease and Epidemic Outbreak Tracking, Communication and Training, Education and Awareness, and Others.
Trends in the route of administration:
- Rescue medications for status epilepticus may be administered via rectal, nasal, or buccal routes, such as diazepam or midazolam.
- The main anti-seizure medications (ASMs) used in this syndrome are typically administered orally.
In 2024, the oral segment accounted for the highest market share in the overall Dravet syndrome market.
- Oral administration of medication is a convenient, cost-effective, and most commonly used medication administration route.
- Medications indicated for the management of this syndrome including tablets, capsules, and oral solutions are administered in the body via the oral route. This type of drug administration is associated with several advantages such as non-invasiveness, patient compliance, and convenience of administration.
- Additionally, oral medications are relatively inexpensive and safer compared to other administration routes such as various invasive methods.
- Developments are made in the various oral drugs to enhance the properties of the medication.
- Fintepla, an oral drug, is in its 3rd trial and is found to reduce seizures in children with hard-to-treat Dravet.
- Consequently, the benefits provided by the oral route of administration are fueling the segment expansion.
The injectable segment is expected to hold the fastest CAGR over the forecast period.
- Several injectable medications for this syndrome are typically administered intravenously and subcutaneously in the body.
- Intravenous administration medications increase bioavailability and also improve patient safety, as they can deliver medications rapidly and precisely making them an essential tool in modern medical practice, particularly in critical care and emergencies
- Moreover, as per the analysis, increasing advances in technology such as enabling the administration of viscous and high-volume biologics through innovations such as VapourSoft® technology for injectable drugs promote the segment trend.
By End Use:
The end-user segment is categorized into hospitals, clinics, and home care settings.
Trends in the End Use:
- There is a growing trend toward home-based care and management of this Syndrome, as it allows patients to receive treatment in a familiar, comfortable environment.
In 2024, the hospital's segment accounted for the highest market revenue share in the overall Dravet syndrome market.
- Hospitals assist as the primary providers of diagnosis and treatment.
- They play a major and crucial role in helping patients by providing convenient, affordable, and easy access to treatment solutions for the syndrome.
- Hospitals are often equipped with state-of-the-art healthcare infrastructure, advanced treatment facilities, and medical personnel required for the better diagnosis and management of this type of syndrome.
- Furthermore, the accelerating rise in the hospital sector is fueling the provision of advanced treatment for this syndrome management which is further boosting the segment expansion.
- As per the analysis, the data published by Invest India, in June 2024 stated that the hospital sector in India was valued at USD 94.97 Billion in FY21 in terms of revenue & is expected to reach 219.44 Billion by FY 2027, growing at a CAGR of 18.24%.
- Consequently, the rising incidence of this syndrome has further elevated the importance of hospitals in its treatment, thereby driving the segment's expansion.
The home care settings segment is expected to hold the fastest CAGR over the forecast period.
- Home care plays an important role for children with this syndrome by offering access to treatment and management for this condition in a familiar, comfortable environment.
- Additionally, home care providers offer several benefits to people with this syndrome, including personalized care, convenience, and flexibility.
- Similarly, the escalating rise in the spending for home care services is a significant factor that is expected to propel the expansion of the segment.
- According to the data published by the Centers for Medicare & Medicaid Services in December 2023, spending for services provided by freestanding home healthcare agencies increased by 6.0% in 2022 to USD 132.9 billion, accelerating from the expansion of 0.3% in 2021.
- As a result, owing to the rise in the spending for home care settings the segment is expected to gain potential expansion globally.
By Distribution Distribution Channel:
The distribution channel segment is categorized into offline and online pharmacies.
Trends in the Distribution Channel:
- Government hospitals serve as an affordable and accessible distribution channel for individual and therapeutic products, implicitly driving overall Dravet syndrome market trends.
- Retail pharmacies have cemented their role as integral members of the healthcare team by becoming one of the most readily available healthcare providers in local communities.
In 2024, the offline segment accounted for the largest market in the overall Dravet syndrome market share.
- The offline segment is further categorized into hospital pharmacies and retail pharmacies.
- These pharmacies are easy to access by hospital staff and patients alike and provide patients with information and support on how to use medications safely and effectively.
- Various standalone pharmacy retail stores are being introduced to allow customers to visit stores to purchase their medications with appropriate guidance.
- By providing convenient, affordable, and accessible services to treatment solutions, these pharmacies help patients live healthier and more fulfilling lives.
- Additionally, they provide an inclusive range of products besides including novel drugs which further improves access to emerging treatment for managing seizures.
- In January 2023, Reliance Retails opened its standalone pharmacy store in Chennai to offer various types of medication including medications to consumers.
- The high benefits and easy availability of this syndrome medications across hospital pharmacy stores are propelling the segment's expansion worldwide.
The online pharmacies segment is expected to hold the fastest CAGR over the forecast period.
- Online pharmacies provide a platform for customers to purchase medicinal drugs and E-services online, allowing the customer to receive medicines/services in the comfort of their homes within a short time.
- Digitalization in the healthcare system and the increasing adoption of e-services are expected to boost the online pharmacy segment in the upcoming years.
- Additionally, the rising e-pharmacy sector is expected to contribute to this expansion trajectory.
- According to the data provided by the India Brand Equity Foundation in July 2023, estimates indicate that the e-pharmacy market size in India accounted for USD 0.5 billion in 2019, accounting for 2-3% of the total Indian pharmacy sales. The market is predicted to increase at a compounded annual rate of 44% to USD 4.5 billion by 2025.
- Thus, the rising e-pharmacy sector is expected to propel the segment expansion in upcoming years.
Regional Analysis:
The regional segment includes North America, Europe, Asia Pacific, the Middle East and Africa, and Latin America.
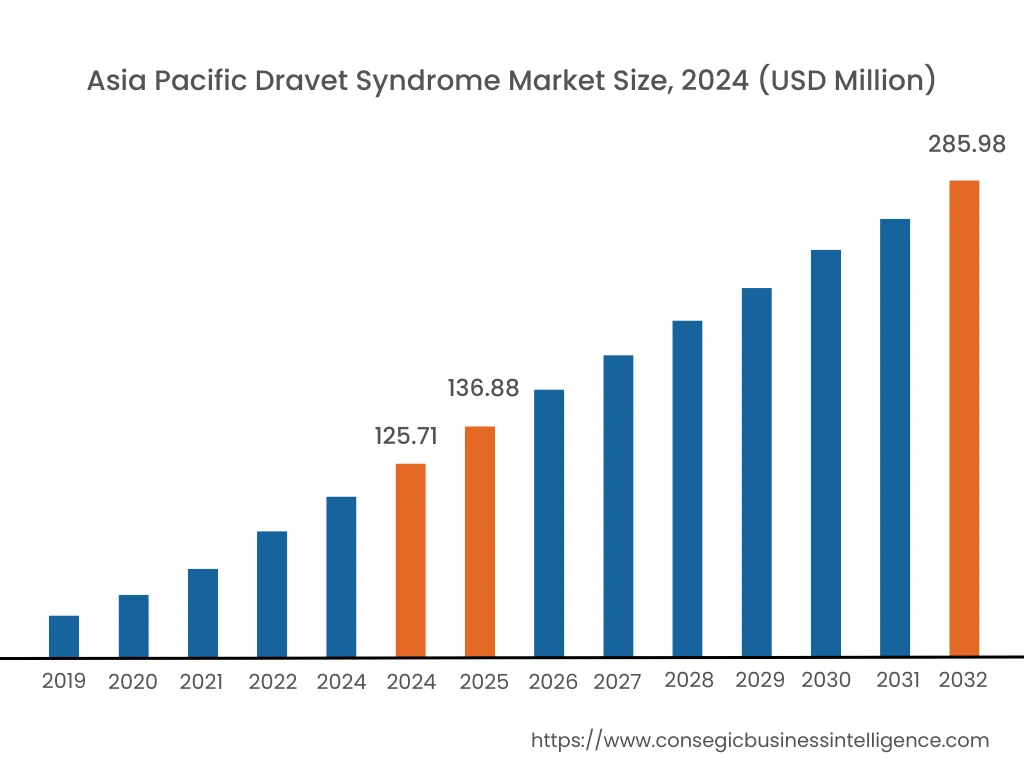
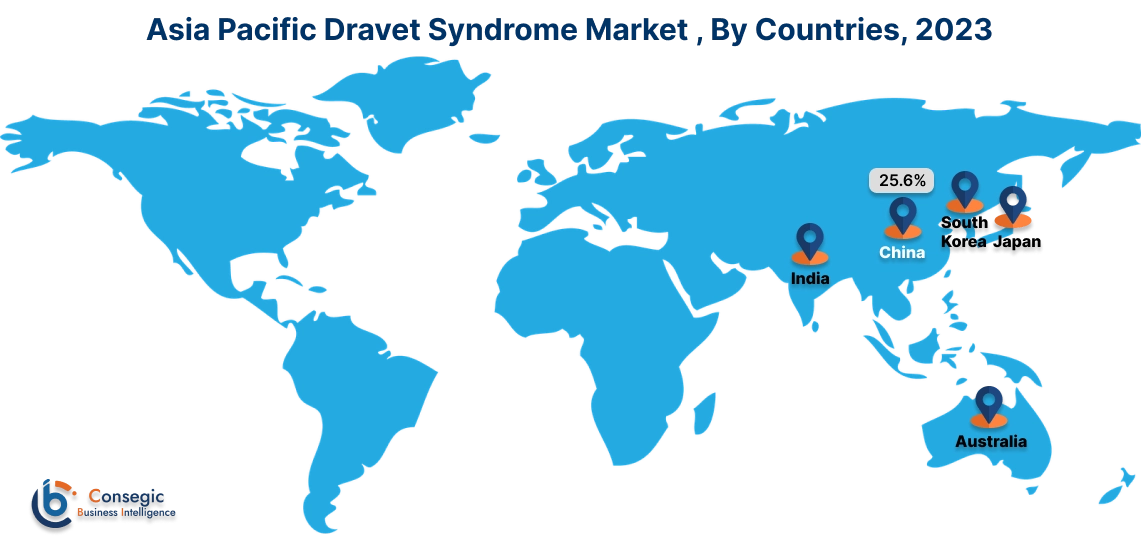
Asia Pacific region is expected to witness significant growth over the forecast period, growing at a CAGR of 10.80% during 2025-2032. In Asia Pacific, China accounted for a major market revenue of 25.6% in 2024.
The significant growth in healthcare spending, better health awareness, and potential growth in the healthcare and pharmaceutical sector across the region are expected to increase the treatment facilities for this syndrome across the region.
- For instance, according to the report published by India Brand Equity Foundation, in August 2023, stated that the market size of the Indian pharmaceuticals industry is expected to reach USD 65 billion by 2024, and approximately USD 130 billion by 2030.
All the above-mentioned factors are collectively driving the demand for this syndrome management from the pharmaceutical and healthcare industry in the Asia Pacific region and creating lucrative Dravet syndrome market opportunities.
North America accounted for the highest Dravet syndrome market share valued at USD 141.59 Million in 2024 and it is expected to reach USD 304.39 Million in 2032. The Dravet syndrome market across the North American region is growing owing to the high prevalence of the disease, the availability of advanced medical facilities, and the presence of well-established healthcare systems across the region. Significant growth in healthcare expenditure is driving the Dravet syndrome market growth in the region.
- Additionally, according to the data published by the Centers for Medicare & Medicaid Services in December 2023, U.S. healthcare spending grew 4.1% to reach USD 4.5 trillion in 2022, faster than the increase of 3.2% in 2021.
Moreover, North America is home to several pharmaceutical companies actively involved in researching and developing new and improved treatments for this syndrome. Thus, as per the analysis, the growing pharmaceutical research and development spending and government healthcare spending across the region are driving market growth.
As per the Dravet Syndrome market analysis, Europe is anticipated to witness substantial growth that is backed by the increasing prevalence of this syndrome. Companies invest in innovative technologies to cater to the surging Dravet syndrome market demand for these treatments across the region.
The Middle East, Africa, and Latin America are expected to grow at a considerable rate due to increasing investments in medical sectors in countries like Brazil, and UAE, among others.
Top Key Players & Market Share Insights:
The global Dravet syndrome market is highly competitive, with several large players and numerous small and medium-sized enterprises. These companies have strong research and development capabilities and a strong presence in the market through their extensive product portfolios and distribution networks. The Dravet syndrome industry is characterized by intense competition, with companies focusing on expanding their product offerings and increasing their industry revenue through mergers, acquisitions, and partnerships. The key players in the Dravet syndrome industry include-
- Stoke Therapeutics(England)
- UCB, Inc. (Belgium)
- Lundbeck (Denmark)
- Harmonay Biosciences(U.S)
- Sanofi(France)
- Biocodex, Inc. (France)
- Jazz Pharmaceuticals, Inc.(Ireland)
- Otter Pharmaceuticals (Subsidiary of Assertio Holdings, Inc.)(U.S)
- AbbVie(U.S)
- Ovid Therapeutics, Inc.(U.S)
Recent Industry Developments :
Merger & Acquisition:
- In January 2022, UCB, a global biopharmaceutical leader focused on creating valuable solutions, announced the acquisition of Zogenix. The total transaction value of the acquisition is up to approximately USD 1.9 billion. Transaction broadens and builds upon UCB's role as a leader in, and our continued commitment to, addressing unmet needs of people living with epilepsy and adds treatment options for specific, vulnerable patient populations with FINTEPLA C-IV oral solution which is approved for seizures associated with Dravet syndrome.
- In March 2021, Takeda Pharmaceutical Company Limited and Ovid Therapeutics Inc., a biopharmaceutical company committed to developing medicines for rare neurological diseases, announced an exclusive agreement under which Takeda will secure global rights at closing from Ovid to develop and commercialize the investigational medicine ‘soticlestat' for the treatment of developmental and epileptic encephalopathies, including Dravet syndrome. Under the agreement, Ovid will receive an upfront payment of USD 196 million at closing and is eligible to receive up to an additional USD 660 million upon achieving development, regulatory, and sales milestones.
Product Approval:
- According to the data published in February 2024, reported that, Encoded Therapeutics has received clearance from the US Food and Drug Administration (FDA) for its Investigational New Drug (IND) application and approval under the Clinical Trial Approval (CTA) scheme from the Australian Therapeutic Goods Administration to initiate clinical trials of its gene therapy candidate, ETX101, for the treatment of SCN1A+ Dravet syndrome.
Dravet Syndrome Market Report Insights :
| Report Attributes | Report Details |
| Study Timeline | 2019-2032 |
| Market Size in 2032 | USD 939.17 Million |
| CAGR (2025-2032) | 10.4% |
| By Treatment |
|
| By Route of Administration |
|
| By End Use |
|
| By Distribution Channel |
|
| By Region |
|
| Key Players |
|
| North America | U.S. Canada Mexico |
| Europe | U.K. Germany France Spain Italy Russia Benelux Rest of Europe |
| APAC | China South Korea Japan India Australia ASEAN Rest of Asia-Pacific |
| Middle East and Africa | GCC Turkey South Africa Rest of MEA |
| LATAM | Brazil Argentina Chile Rest of LATAM |
| Report Coverage |
|
Key Questions Answered in the Report
How big was the Dravet syndrome market in 2024? +
In 2024, the market size of Dravet syndrome was USD 426.84 million.
What is the fastest-growing region in the Dravet Syndrome market? +
The Asia Pacific region is the fastest-growing region.
What specific segmentation details are covered in the Dravet Syndrome report? +
The segment details covered consist of treatment, route of administration, end-use, and distribution channel.
Which are the key players in the Dravet Syndrome Market? +
The key players include Stoke Therapeutics, UCB, Inc., Biocodex, Inc., Jazz Pharmaceuticals, Inc., Lundbeck, Epygenix Therapeutics, Inc., Sanofi, Otter Pharmaceuticals (Subsidiary of Assertio Holdings, Inc.), AbbVie, and Ovid Therapeutics, Inc.