- Summary
- Table Of Content
- Methodology
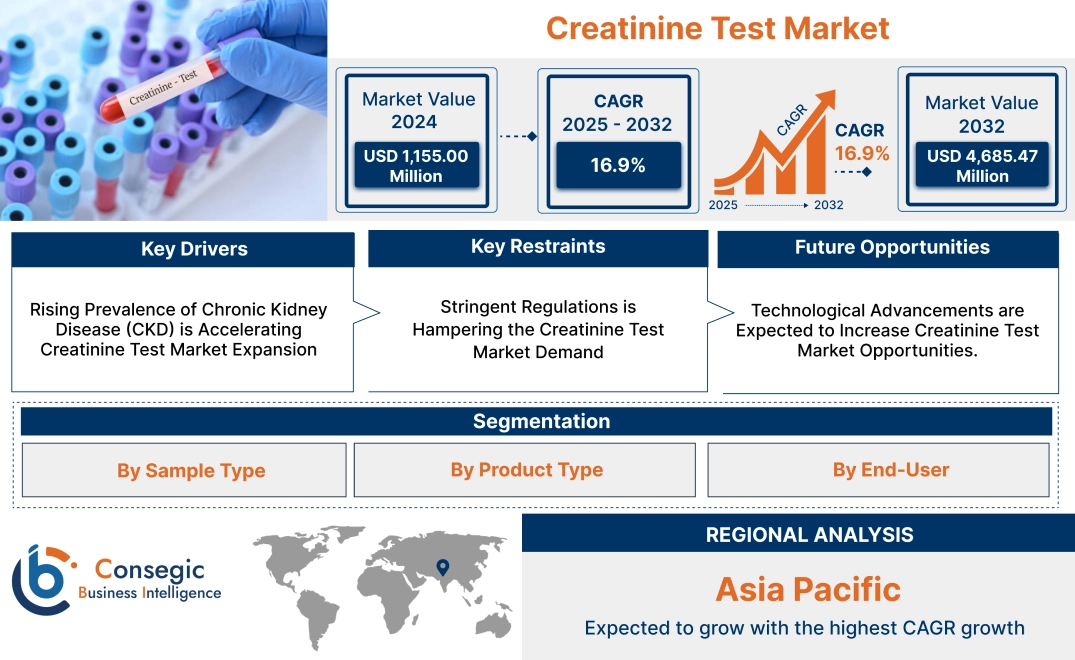
Creatinine Test Market Size:
Creatinine Test Market size is growing with a CAGR of 16.9% during the forecast period (2025-2032), and the market is projected to be valued at USD 4,685.47 Million by 2032 from USD 1,360.44 Million in 2024.
Creatinine Test Market Scope & Overview:
A creatinine test measures the level of creatinine, a waste product produced by muscle metabolism, in the body. This test is performed on blood (serum creatinine) or urine, with each offering specific insights into kidney health. The tests utilize various product types. Test kits provide all necessary components in convenient packages, while instruments are specialized devices for analyzing creatinine levels in samples. Reagents are the chemical substances that interact with creatinine in the sample to produce a measurable signal, enabling the quantification of creatinine levels. This test is widely used in various medical applications. Primarily, they are crucial for diagnosing and monitoring chronic kidney disease (CKD) by assessing kidney function. Additionally, these tests help evaluate kidney function in other medical conditions, monitor drug toxicity, assess heart failure, evaluate transplant function, and monitor muscle disorders.
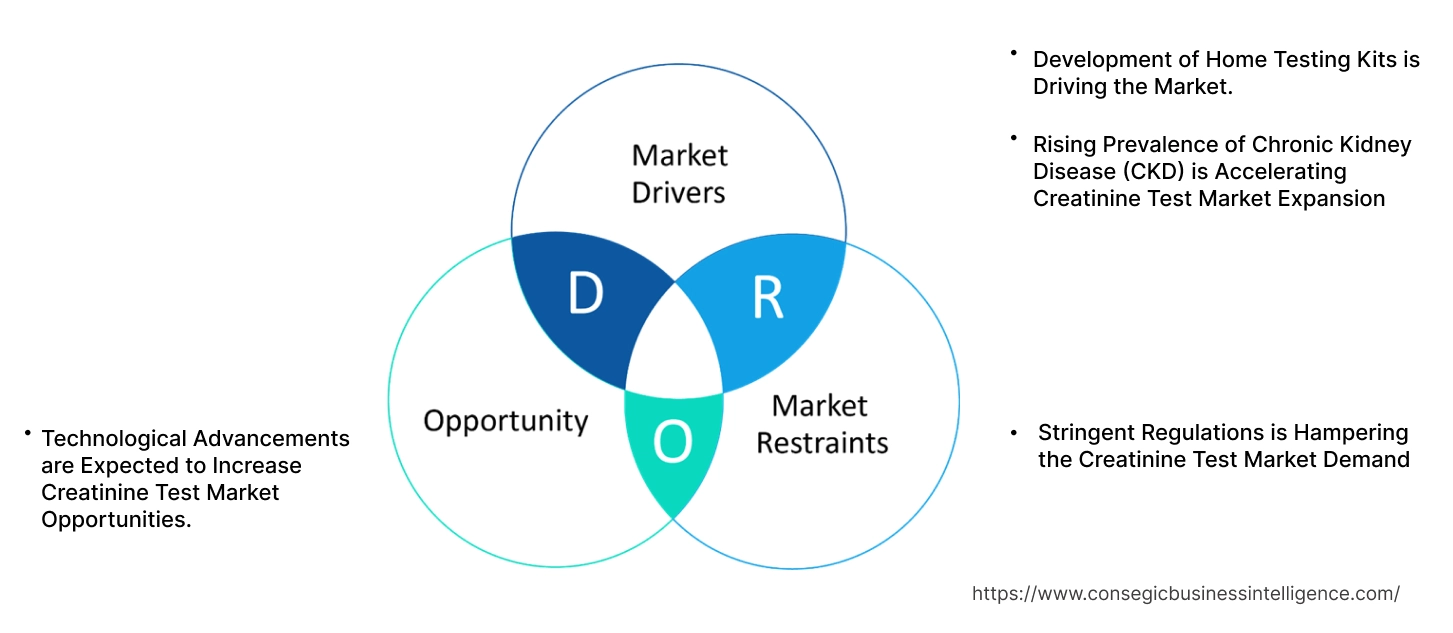
Key Drivers:
Rising Prevalence of Chronic Kidney Disease (CKD) is Accelerating Creatinine Test Market Expansion
Chronic kidney disease (CKD) is a condition where the kidneys are gradually damaged and lose their ability to filter waste products from the blood. This leads to a buildup of toxins in the body and other serious health problems. Creatinine test plays a critical role in diagnosing and managing chronic kidney diseases by measuring serum creatinine levels, an indicator of kidney function.
Elevated creatinine levels signify impaired kidney filtration efficiency, helping detect CKD at an early stage. Regular monitoring through this test aids in tracking disease progression, evaluating treatment efficacy, and preventing complications such as kidney failure. The rising prevalence of diseases such as diabetes and hypertension has contributed to increased cases of CKD.
For instance,
- According to the S. Renal Data System 2023 Annual Report, over 808,000 Americans are living with end-stage renal disease (ESKD), which is the most deadly stage of CKD. This has underscored the critical need for regular testing of creatinine for monitoring kidney function in patients, positively influencing the creatinine test market trends.
Overall, the high prevalence of chronic kidney disease is significantly boosting the creatinine test market expansion.
Development of Home Testing Kits is Driving the Market.
Home testing kits typically offer user-friendly designs, such as test strips or portable devices, allowing individuals to measure creatinine levels in urine or blood. Moreover, advancements in biosensor technology and miniaturized diagnostic tools have significantly enhanced their accuracy and reliability.
- In October 2023, Everly Health introduced a convenient at-home kidney health test that combines an estimated glomerular filtration rate (eGFR) obtained from a blood sample to evaluate kidney function, along with a urinary albumin-to-creatinine ratio (UACR) measured through urine collection to further assess kidney health. This convenience is particularly beneficial for patients with CKD, diabetes, or hypertension who require regular monitoring to manage their conditions effectively, positively influencing creatinine test market trends.
Additionally, home testing kits are also gaining traction in rural and remote areas where access to healthcare facilities is limited. These kits reduce the burden on healthcare infrastructure while empowering patients to take control of their health. Furthermore, these kits align with the growing trend toward preventive healthcare, enabling early detection of kidney dysfunction and proactive intervention.
Overall, the development of user-friendly and accurate home testing kits, coupled with their increasing accessibility, is accelerating the global creatinine test market growth.
Key Restraints:
Stringent Regulations is Hampering the Creatinine Test Market Demand
Stringent regulations pose a significant restraint for the market, particularly for instruments and testing kits. Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) enforce strict guidelines to ensure the safety, accuracy, and reliability of diagnostic tools.
Manufacturers face significant hurdles in meeting these stringent requirements, including extensive clinical trials, quality control measures, and documentation. This increases the cost and complexity of product development, which discourages innovation, particularly for smaller companies with limited resources. Non-compliance with these regulations results in recalls, legal penalties, and damage to the manufacturer's reputation.
Additionally, variations in regulatory standards across regions create further challenges for companies looking to expand globally. Furthermore, compliance with these regulations requires continuous monitoring and periodic updates, adding to operational costs. The need for approvals and certifications slows down the availability of advanced diagnostic technologies in the market, limiting their accessibility to patients.
Overall, analysis shows that the stringent regulatory requirements significantly increase the cost and complexity of developing and commercializing new testing products, hampering the creatinine test market demand.
Future Opportunities :
Technological Advancements are Expected to Increase Creatinine Test Market Opportunities.
Technological advancements are driving significant growth in the market, enhancing diagnostic accuracy, efficiency, and accessibility. Automation in laboratory instruments has also revolutionized testing for creatinine. Automated analyzers have streamlined sample processing, reduced manual errors, and increased throughput, making them ideal for high-volume testing in hospitals and diagnostic centers. Moreover, nanotechnology is an emerging field with the potential to revolutionize creatinine detection.
- According to a study published in ScienceDirect, in 2024, nanomaterials are enhancing creatinine detection in biosensors by improving electron transfer rates between enzymes and electrodes. Surface modifications create selective membranes, minimizing interference. Additionally, color changes in nanoparticle solutions upon creatinine addition enable detection. These advancements hold promises for early CKD diagnosis, creating potential for the market.
Additionally, artificial intelligence (AI) and machine learning (ML) are also emerging as transformative technologies. By analyzing large datasets, AI tools enhance diagnostic accuracy, predict disease progression, and optimize treatment plans. These advancements are reshaping the market, enabling early detection, personalized care, and improved health outcomes.
Overall, technological advancements, including automation, nanotechnology, and AI are expected to increase creatinine test market opportunities.
Creatinine Test Market Segmental Analysis :
By Sample Type:
Based on sample type, the market is categorized into blood and urine.
Trends in Sample Type:
- Dried blood spot (DBS) testing is emerging as a promising alternative.
- Advancements in urine collection methods are improving accuracy.
The blood segment accounted for the largest market share in 2024.
- Measuring serum creatinine levels through blood tests is considered the gold standard for evaluating kidney function.
- It provides precise data critical for calculating the glomerular filtration rate (eGFR). This metric is a cornerstone in diagnosing and monitoring chronic kidney diseases and other renal disorders, enabling early detection and effective disease management.
- Moreover, blood tests are highly standardized and widely accepted in clinical settings, offering consistent results across diverse patient populations. Advanced laboratory instruments and automated analyzers are enhancing the accuracy and efficiency of these tests.
- For instance, in 2022, Nova Biomedical launched the innovative Nova Max Pro Creatinine/eGFR Meter System, which accurately measures blood creatinine levels using only a minuscule 1.2 microliter capillary fingerstick blood sample in a mere 30 seconds. This launch of new devices has increased accessibility, driving the segment.
- Overall, as per the market analysis, blood tests remain the gold standard for testing. Advancements in devices are driving the segment in the global creatinine test market growth.
The urine segment is expected to grow at the fastest CAGR over the forecast period.
- Urine-based testing is emerging due to its convenience, non-invasive nature, and growing relevance in preventive and home-based healthcare. These tests are particularly useful for measuring creatinine clearance, which evaluates the kidneys' ability to filter waste products.
- As they do not require blood collection, urine tests are less intimidating for patients and are more accessible for routine monitoring, especially in outpatient and at-home settings.
- Moreover, a urine test helps detect kidney problems, such as kidney disease, kidney infection, kidney failure, kidney stones, and urinary tract obstruction.
- As per the market analysis, the growing need for non-invasive and convenient testing options is driving segmental growth for the forecasted years.
By Product Type:
The product type segment is categorized into instruments and consumables.
Trends in the Product Type:
- Focus on developing user-friendly and easy-to-use home testing kits.
- Increased need for point-of-care (POC) testing devices.
The consumables segment accounted for the largest market share of 2024 and is expected to grow at the fastest CAGR over the forecast period.
- Consumables are further categorized into test kits, reagents, cartridges, and others. Test kits are pre-packaged sets containing all necessary components for a specific test. Reagents are chemical substances that interact with creatinine in the sample to produce a measurable signal. Cartridges are pre-filled containers for automated systems.
- They are essential for every test performed, regardless of the instrument or method used. Moreover, their single-use nature necessitates regular replenishment, leading to consistent requirements and segment dominance.
- There is a growing number of kidney-related disorders and a rising need for home-based testing and point-of-care testing (POCT).
- For instance, according to Express Healthcare, individuals in India have increased awareness, and the market for POCT is expected to grow by a CAGR of 17.3 percent between 2022 and 2027. This has impacted the market by increasing the need for consumables such as test kits and cartridges, thus driving the segment. This convenience supports their growing popularity among patients and healthcare providers.
- Overall, as per the market analysis, the rising prevalence of kidney diseases, coupled with the growing demand for POCT and home testing, is driving segmental growth in the creatinine test industry.
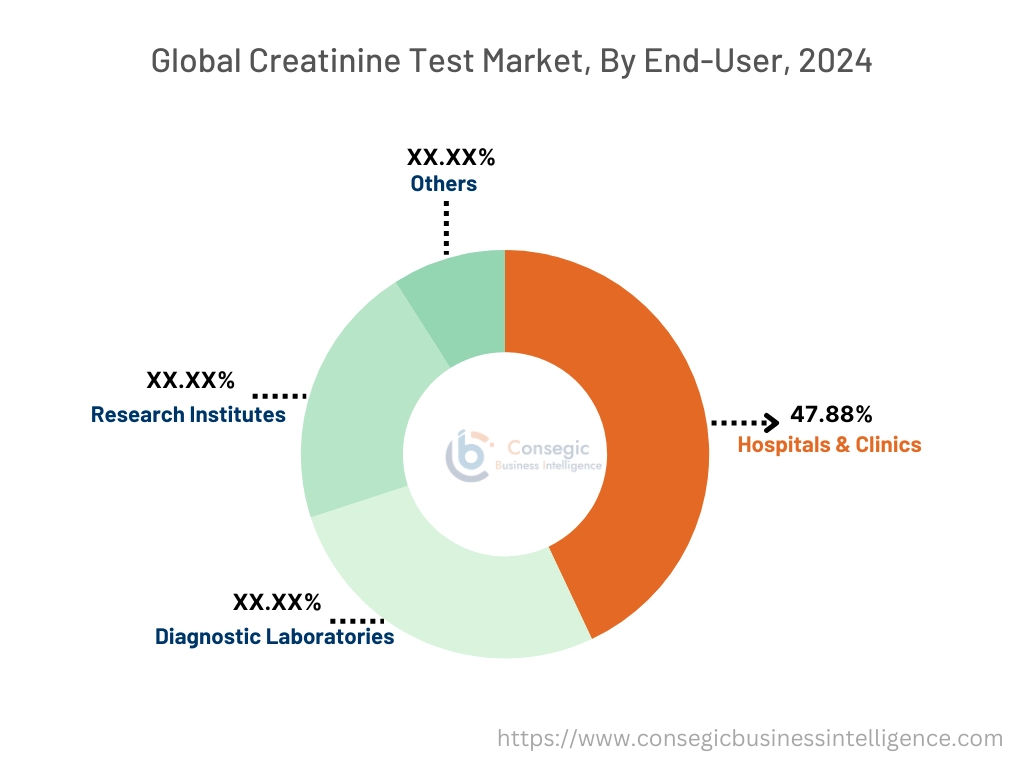
By End-User:
The end-user segment is categorized into hospitals & clinics, diagnostic laboratories, research institutes, and others.
Trends in End-User:
- Home healthcare is gaining traction, driving needs for home-based testing.
- Diagnostic laboratories are emerging as key players, offering convenient and accessible testing services.
The hospitals & clinics segment accounted for the largest market share of 47.88% in 2024.
- Hospitals and clinics dominate as these healthcare settings manage a large number of patients, necessitating frequent and comprehensive diagnostic testing, including creatinine assessments. This high patient turnover leads to a consistent demand for testing services.
- Moreover, hospitals and clinics are equipped with sophisticated laboratory infrastructure and skilled personnel capable of performing complex diagnostic procedures. This enables them to offer a wide range of tests, including creatinine measurements, with high accuracy and reliability.
- Additionally, in these institutions’ creatinine testing is part of routine check-ups, pre-surgical evaluations, and monitoring of chronic conditions, ensuring continuous patient care. Rising healthcare industry in terms of increased investment, technological advancements, growing diseases, and government regulations have led to increases in hospitals and clinics.
- For instance, according to CEIC, the number of hospitals in China increased by 3.85% from 2022 to 2023, rising from 36 million units to 38 million units. Consequently, this surge in hospitals has contributed to a higher volume of diagnostics and treatments related to kidney diseases, leading to an increased need for creatinine assessments, positively driving the segment in the market.
- Overall, high patient volume, advanced infrastructure, and the integration of creatinine measurements into routine care contribute to the dominance of the segment. Rising numbers of hospitals and clinics globally are driving the segment further.
The diagnostic laboratories segment is expected to grow at the fastest CAGR over the forecast period.
- Diagnostic laboratories are emerging as key players in the market due to several contributing factors. Diagnostic laboratories focus specifically on testing and diagnostics, making them highly efficient at conducting a wide range of medical tests, including creatinine assessments.
- This specialization allows them to cater to a growing patient need for kidney function monitoring, particularly in urban areas where access to hospitals is limited.
- Moreover, compared to hospitals, diagnostic centers tend to have lower operational costs. This makes them more affordable for patients who require routine or preventive testing.
- With the rise in chronic kidney disease (CKD) awareness, more individuals are opting for affordable testing options at diagnostic centers, contributing to segmental growth.
- Furthermore, these centers are increasingly offering accessible locations and shorter wait times, attracting patients who seek quick results without the long delays typical of hospitals. Many diagnostic centers also provide home testing services, further increasing their appeal.
Regional Analysis:
The regional segment includes North America, Europe, Asia Pacific, the Middle East and Africa, and Latin America.
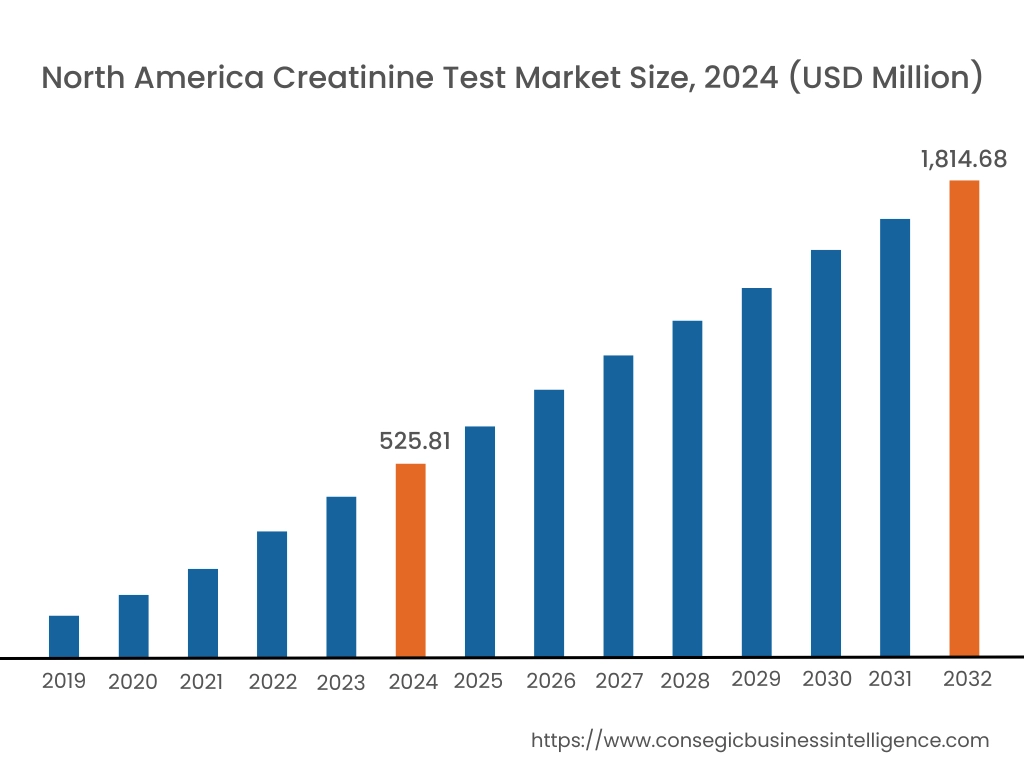
In 2024, North America accounted for the highest creatinine test market share at 38.65% and was valued at USD 525.81 Million and is expected to reach USD 1,814.68 Million in 2032. In North America, the U.S. accounted for the highest creatinine test market share of 74.11% during the base year of 2024. North America, particularly the United States and Canada, has a well-established healthcare system with advanced diagnostic technologies and infrastructure. Hospitals, clinics, and diagnostic centers in this region are equipped with state-of-the-art instruments and technologies, allowing for accurate, efficient, and high volume of testing for creatinine.
- According to an article published in Gentian Diagnostics, in 2023, the United States conducts an estimated 400,000 to 500,000 creatinine tests annually, positively influencing the market.
Moreover, the availability of high-quality medical facilities supports the high demand for kidney function testing. Additionally, the rising prevalence of CKD, driven by factors such as obesity, diabetes, and hypertension, is a significant contributor to the dominance of North America in the market. Furthermore, North American countries, particularly the U.S., offer favorable reimbursement policies for diagnostic tests, making testing more accessible to a wider population. These policies help ensure a steady need for testing services in hospitals, clinics, and diagnostic centers. Overall, advanced healthcare infrastructure, high prevalence of CKD, and favorable reimbursement policies contribute to North America's dominance in market
In Asia Pacific, the creatinine test market is experiencing the fastest growth with a CAGR of 17.4% over the forecast period. Governments in APAC countries are heavily investing in healthcare infrastructure, improving access to diagnostics in both urban and rural areas. Initiatives to expand universal healthcare coverage in countries such as China and India are facilitating widespread adoption of routine diagnostic testing, positively driving the market. Moreover, rapid urbanization and economic development in APAC have increased disposable incomes, enabling more people to afford healthcare services. This trend is boosting the need for preventive healthcare and diagnostic testing, including creatinine assessments. Additionally, the presence of regional manufacturers producing affordable consumables and instruments caters to cost-sensitive markets, enhancing accessibility and market penetration for testing solutions in APAC.
Europe's creatinine test market analysis states that several trends are responsible for the progress of the market in the region. The market is driven by its advanced healthcare systems and aging population. The region has a high prevalence of chronic diseases such as hypertension and diabetes, which are leading causes of chronic kidney disease. Countries such as Germany, the UK, and France dominate due to their robust diagnostic infrastructure and widespread adoption of advanced testing technologies. Moreover, the presence of well-established diagnostic companies in Europe fosters innovation in testing kits and instruments, enhancing accuracy and ease of use. Additionally, increasing public awareness about CKD through educational campaigns drives the need for regular kidney function testing. However, economic disparities between Western and Eastern Europe create varied market penetration.
The Middle East and Africa (MEA) creatinine test market analysis states that the region is also witnessing a notable surge. Government initiatives to improve healthcare systems, particularly in Saudi Arabia, and the UAE are enhancing access to diagnostic services. Investments in modern diagnostic facilities and partnerships with global healthcare providers are boosting the availability of testing for creatinine. Moreover, in Africa, the focus is on addressing unmet medical needs, with international aid and non-governmental organizations supporting diagnostic services. Portable and cost-effective point-of-care testing solutions are gaining traction, further driving the market. However, challenges such as economic constraints and limited awareness hamper the market in the region.
Latin America's creatinine test market size is also emerging. Countries such as Brazil, Mexico, and Argentina lead the market owing to their larger populations and improved healthcare infrastructure. Moreover, urbanization and lifestyle changes have escalated rates of diabetes and hypertension, major contributors to CKD. As awareness of kidney health grows, the need for routine testing of creatinine is rising in both urban and semi-urban regions. Additionally, Brazil, the largest market in the region, benefits from its universal healthcare system, which includes diagnostics for kidney function. Meanwhile, Mexico's proximity to North America drives the adoption of advanced diagnostic technologies through partnerships with global companies. Economic challenges and disparities in healthcare access across countries remain hurdles.
Top Key Players and Market Share Insights:
The Creatinine Test market is highly competitive with major players providing products to the national and international markets. Key players are adopting several strategies in research and development (R&D) and product innovation to hold a strong position in the global Creatinine Test market. Key players in the Creatinine Test industry include-
- Abbott Laboratories (U.S.)
- Hoffmann-La Roche Ltd (Switzerland)
- Chondrex, Inc. (U.S.)
- Great Lakes Dental Technologies (U.S.)
- FUJIFILM Wako Pure Chemical Corporation (Japan)
- Thermo Fisher Scientific Inc. (U.S.)
- Abcam Plc (UK)
- BioAssay Systems (U.S.)
- Sysmex Corporation (Japan)
- Arbor Assays (U.S.)
Recent Industry Developments :
Product Expansion:
- In May 2024, Nova Biomedical declared that the FDA has granted 510(k) clearance for the micro capillary sample mode on the Stat Profile Prime Plus Critical Care analyzer. The Stat Profile Prime Plus offers the latest and most clinically effective critical care tests, including creatinine.
Creatinine Test Market Report Insights :
| Report Attributes | Report Details |
| Study Timeline | 2019-2032 |
| Market Size in 2032 | USD 4,685.47 Million |
| CAGR (2025-2032) | 16.9% |
| By Sample Type |
|
| By Product Type |
|
| By End-User |
|
| By Region |
|
| Key Players |
|
| North America | U.S. Canada Mexico |
| Europe | U.K. Germany France Spain Italy Russia Benelux Rest of Europe |
| APAC | China South Korea Japan India Australia ASEAN Rest of Asia-Pacific |
| Middle East and Africa | GCC Turkey South Africa Rest of MEA |
| LATAM | Brazil Argentina Chile Rest of LATAM |
| Report Coverage |
|
Key Questions Answered in the Report
How big is the Creatinine Test market? +
In 2024, the Creatinine Test market is USD 1,360.44 Million.
Which is the fastest-growing region in the Creatinine Test market? +
Asia Pacific is the fastest-growing region in the Creatinine Test market.
What specific segmentation details are covered in the Creatinine Test market? +
Sample Type and Product Type segmentation details are covered in the Creatinine Test market
Who are the major players in the Creatinine Test market? +
Abbott Laboratories (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), Thermo Fisher Scientific Inc. (U.S.), Abcam Plc (UK), BioAssay Systems (U.S.), Sysmex Corporation (Japan), Arbor Assays (U.S.), Chondrex, Inc. (U.S.), Great Lakes Dental Technologies (U.S.), and FUJIFILM Wako Pure Chemical Corporation (Japan).