- Summary
- Table Of Content
- Methodology
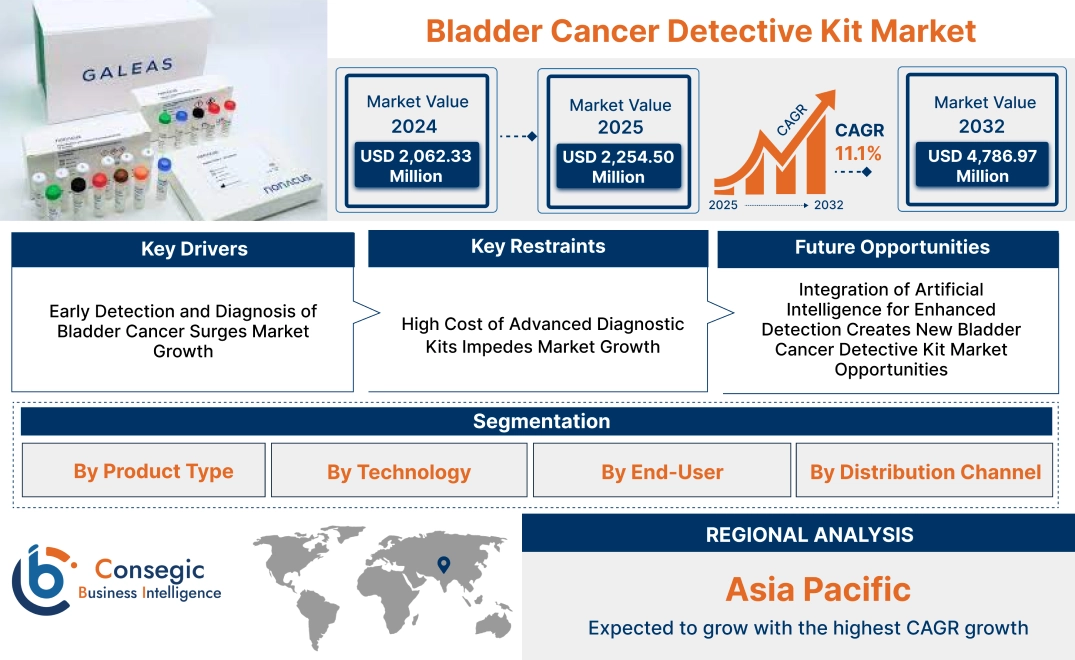
Bladder Cancer Detective Kit Market Size:
Bladder Cancer Detective Kit Market size is estimated to reach over USD 4,786.97 Million by 2032 from a value of USD 2,062.33 Million in 2024 and is projected to grow by USD 2,254.50 Million in 2025, growing at a CAGR of 11.1% from 2025 to 2032.
Bladder Cancer Detective Kit Market Scope & Overview:
Bladder cancer detective kits are diagnostic tools used to identify and detect bladder cancer at early stages. These kits typically consist of urine tests, biomarkers, and advanced detection technology to analyze samples. The kits offer non-invasive, accurate, and quick detection methods. They provide reliable results, aiding early intervention and improved patient outcomes. Their features include high sensitivity, ease of use, and minimal patient discomfort.
Bladder cancer detective kits find applications in hospitals, diagnostic laboratories, and clinics. They are widely used in the healthcare sector to diagnose bladder cancer in patients at risk. These kits contribute significantly to cancer management, early diagnosis, and improved treatment planning.
Key Drivers:
Early Detection and Diagnosis of Bladder Cancer Surges Market Growth
Bladder cancer detection has traditionally relied on invasive procedures, such as cystoscopy. However, advancements in non-invasive diagnostic kits are revolutionizing the way bladder cancer is detected. Bladder cancer detective kits offer a quicker, less painful alternative for diagnosing bladder cancer, often through urine samples. These kits utilize biomarkers and molecular technologies to detect the presence of cancerous cells or abnormal growths in the bladder. For instance, the UroVysion Bladder Cancer Kit, which detects genetic alterations in urine, provides a non-invasive and effective method to identify patients at risk for bladder cancer.
As early detection of bladder cancer significantly increases survival rates and reduces treatment costs, the trend for these diagnostic kits continues to rise, thereby boosting bladder cancer detective kit market growth.
Key Restraints:
High Cost of Advanced Diagnostic Kits Impedes Market Growth
Bladder cancer detective kits, particularly those utilizing cutting-edge molecular diagnostics or biomarker analysis, often come with high production and testing costs. These costs can be prohibitive for healthcare systems, especially in developing regions or for patients without adequate insurance coverage. For example, kits using sophisticated genetic testing or liquid biopsy technologies may not be affordable for widespread use in clinics and hospitals. The high cost of these kits limits accessibility and adoption, especially in low-resource healthcare settings, thus hindering the growth of the market.
The price barriers associated with advanced diagnostic solutions impede the widespread implementation of bladder cancer detective kits in global healthcare.
Future Opportunities:
Integration of Artificial Intelligence for Enhanced Detection Creates New Bladder Cancer Detective Kit Market Opportunities
The integration of artificial intelligence (AI) in bladder cancer diagnostic kits presents a significant opportunity for improving the accuracy and efficiency of early detection. AI technologies can analyze large datasets, identify subtle patterns in test results, and enhance the performance of diagnostic kits. For instance, machine learning algorithms could be applied to urine samples or imaging data, identifying potential cancer biomarkers more accurately and faster than traditional methods.
As AI-powered diagnostic kits are developed, they have the potential to revolutionize bladder cancer detection by increasing accuracy, reducing false positives, and lowering costs. The application of AI in bladder cancer detection is expected to open new avenues for the market in the near future.
Bladder Cancer Detective Kit Market Segmental Analysis :
By Product Type:
Based on product type, the market is segmented into diagnostic kits and monitoring kits.
The diagnostic kits sector accounted for the largest revenue in bladder cancer detective kit market share in 2024.
- Diagnostic kits are used primarily for identifying bladder cancer through various testing methods, enabling early detection and diagnosis.
- These kits typically involve sample collection and analysis using advanced technologies such as immunoassays and molecular diagnostics.
- The increasing prevalence of bladder cancer and the growing trend for early detection and personalized healthcare are key factors influencing the growth of diagnostic kits.
- Moreover, these kits are highly accurate and offer quick results, making them a preferred option for healthcare professionals.
- Therefore, according to bladder cancer detective kit market analysis, diagnostic kits are driving the bladder cancer detective kit market trends due to their high bladder cancer detective kit market demand in early diagnosis and screening procedures.
The monitoring kits sector is anticipated to register the fastest CAGR during the forecast period.
- Monitoring kits are designed to track the progress of the disease in patients who have already been diagnosed with bladder cancer.
- They are used to measure biomarkers, assess treatment efficacy, and detect recurrence, helping to manage the patient's ongoing care.
- The rising focus on personalized treatment and the need for regular monitoring of bladder cancer patients is contributing to the segment’s anticipated growth.
- These kits provide real-time data that helps clinicians adjust treatment plans efficiently.
- Thus, according to bladder cancer detective kit market analysis, monitoring kits are expected to register rapid growth in the market, driven by their growing importance in ongoing disease management.
By Technology:
Based on technology, the market is segmented into immunoassays, molecular diagnostics, and imaging techniques.
Immunoassays accounted for the largest revenue in bladder cancer detective kit market share in 2024.
- Immunoassays are widely used in bladder cancer detection due to their high sensitivity and specificity in identifying biomarkers related to cancer.
- These assays are based on the principle of antigen-antibody reactions, providing accurate and reliable results for the diagnosis of various cancers, including bladder cancer.
- The increasing preference for non-invasive diagnostic methods and advancements in immunoassay technology contribute to their dominance in the market.
- The trend for early detection of bladder cancer and the efficiency of immunoassays in providing quick results further drive their adoption.
- Therefore, according to market analysis, immunoassays are expected to maintain a dominant position in the market due to their proven effectiveness and wide usage.
Molecular diagnostics is expected to register the fastest CAGR during the forecast period.
- Molecular diagnostics involves analyzing the genetic material of cancer cells, providing a highly accurate and precise diagnosis of bladder cancer.
- This technology enables the detection of mutations, gene expression profiles, and other genetic biomarkers that are crucial in identifying cancer at its earliest stages.
- The rising focus on personalized medicine and precision diagnostics is a key driver for the growth of molecular diagnostics.
- These diagnostics offer greater specificity and can be utilized to guide treatment decisions, making them increasingly popular in the healthcare industry.
- Thus, according to market analysis, molecular diagnostics are anticipated to expand rapidly in the market, driven by technological innovations and the growing trend for personalized treatment options.
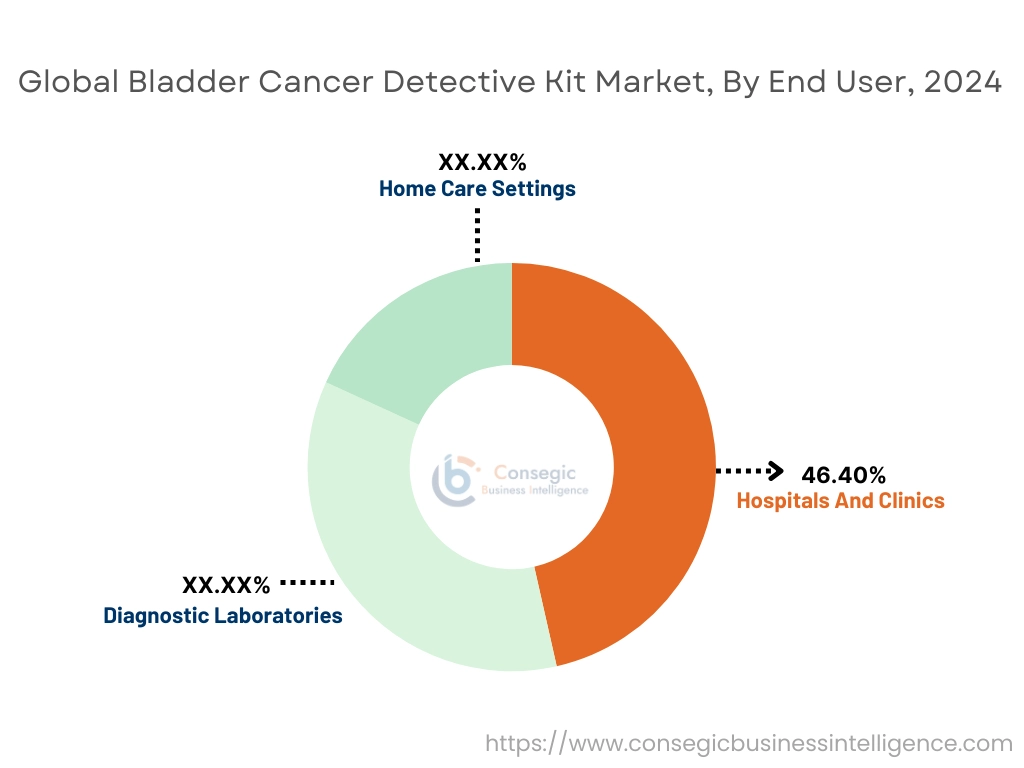
By End-User:
Based on end-users, the market is segmented into hospitals and clinics, diagnostic laboratories, and home care settings.
Hospitals and clinics accounted for the largest revenue share by 46.40% in 2024.
- Hospitals and clinics represent the primary settings where bladder cancer detective kits are used for diagnosis and monitoring of patients.
- These institutions have the necessary infrastructure and expertise to handle sophisticated diagnostic tools, ensuring accurate and efficient results.
- The increasing number of bladder cancer cases and the rising bladder cancer detective kit market demand for early detection services are driving the adoption of these kits in healthcare institutions.
- Additionally, hospitals and clinics play a vital role in the administration of cancer treatments, further boosting the trend for bladder cancer detective kits.
- Therefore, according to market analysis, hospitals and clinics continue to dominate the market due to their large patient base and advanced healthcare facilities.
Diagnostic laboratories are anticipated to register the fastest CAGR during the forecast period.
- Diagnostic laboratories are specialized in providing comprehensive testing services, including cancer detection and monitoring.
- These laboratories offer specialized and highly accurate diagnostic services that cater to a growing trend for quick and precise bladder cancer detection.
- The rise in healthcare outsourcing and the increasing preference for specialized testing services are fueling the growth of diagnostic laboratories in the market.
- Moreover, advancements in laboratory technologies and the availability of highly specialized staff contribute to the growth of this segment.
- Thus, according to market analysis, diagnostic laboratories are expected to grow rapidly in the market, driven by the increasing demand for accurate testing and early detection services.
By Distribution Channel:
Based on distribution channels, the market is segmented into direct sales, distributors, and online platforms.
Direct sales accounted for the largest revenue share in 2024.
- Direct sales involve manufacturers selling bladder cancer detective kits directly to healthcare institutions, ensuring better control over product distribution and customer relationships.
- This model enables manufacturers to provide tailored services, offer product education, and ensure that their products are used effectively in clinical settings.
- Direct sales channels are particularly dominant in established healthcare systems with a strong focus on advanced diagnostic tools and equipment.
- As healthcare institutions increasingly prioritize reliable suppliers, direct sales continue to be a preferred method of distribution for bladder cancer diagnostic kits.
- Therefore, according to market analysis, direct sales remain the leading distribution channel in the bladder cancer detective kit market, providing a streamlined process for delivering products to key end-users.
Online platforms are expected to register the fastest CAGR during the forecast period.
- Online platforms offer convenience and accessibility for purchasing bladder cancer detective kits, particularly for home care settings or small healthcare providers.
- The increasing use of e-commerce in healthcare and the rise of telemedicine are contributing to the growth of online distribution channels.
- Online platforms provide easy access to a wide range of diagnostic kits and allow for quick ordering and delivery, thus expanding the market’s reach.
- Additionally, the rise of home testing kits for cancer detection is further boosting the demand for online platforms in this segment.
- Thus, according to market analysis, the growing bladder cancer detective kit market trend of digital healthcare and consumer preference for online shopping is expected to drive rapid growth in the online platforms distribution channel.
Regional Analysis:
The regional segment includes North America, Europe, Asia Pacific, Middle East and Africa, and Latin America.
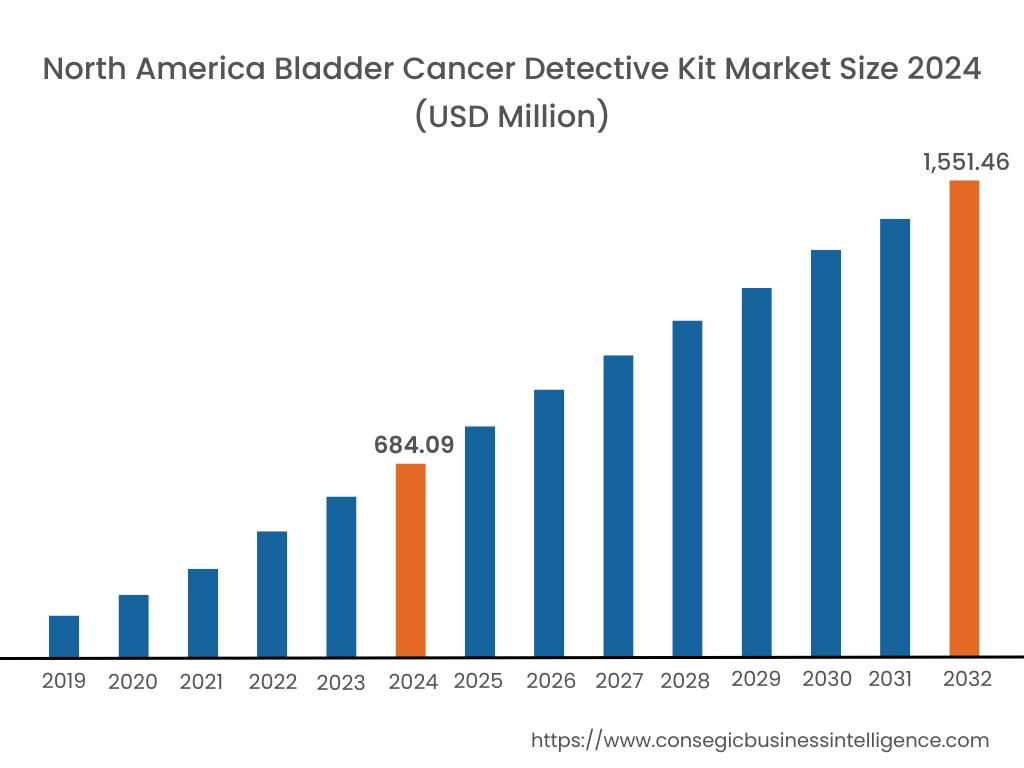
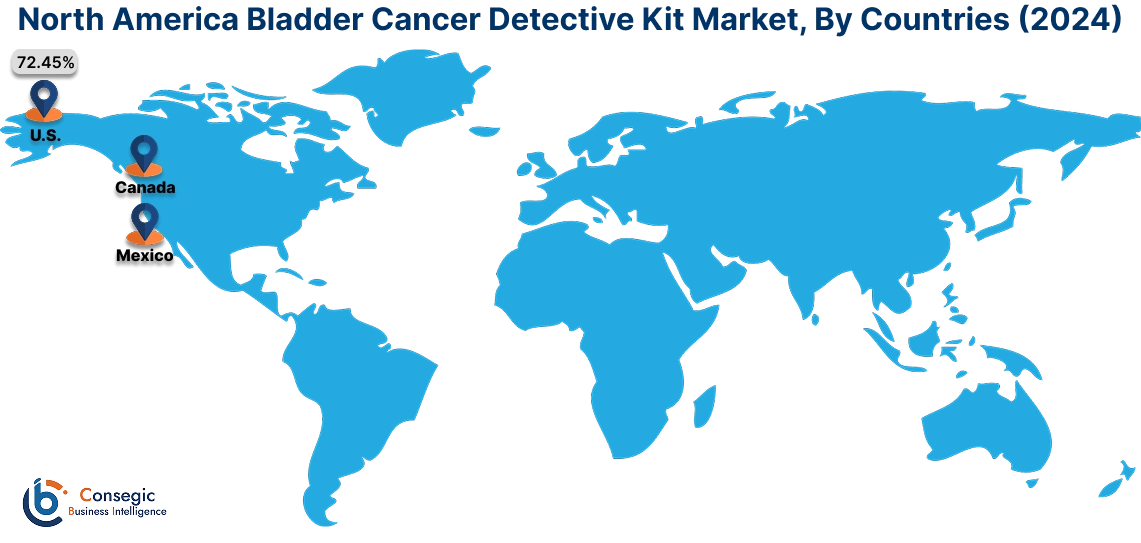
In 2024, North America was valued at USD 684.09 Million and is expected to reach USD 1,551.46 Million in 2032. In North America, the U.S. accounted for the highest share of 72.45% during the base year of 2024. North America holds the largest share of the bladder cancer detective kit market due to advanced healthcare infrastructure and high prevalence of bladder cancer. The United States leads the market with a well-established healthcare system and high awareness of early detection methods. The adoption of innovative diagnostic technologies, including non-invasive kits, is increasing among healthcare providers. Regulatory support for novel diagnostic solutions and reimbursement policies further enhance market performance in this region.
In Asia Pacific, the market is experiencing the fastest growth with a CAGR of 11.6% over the forecast period. The Asia-Pacific region is witnessing rapid bladder cancer detective kit market expansion, driven by rising healthcare awareness and an increasing number of bladder cancer cases. Countries like Japan, China, and India are seeing increased adoption of bladder cancer diagnostic kits due to improving healthcare access and government initiatives. However, challenges remain in rural areas due to limited awareness and healthcare access. Nevertheless, the increasing focus on early detection and improved diagnostic tools by local players and international collaborations boosts market growth in the region.
Europe shows steady demand for bladder cancer detective kits, driven by the region's well-established healthcare system and the growing emphasis on early cancer detection. Major markets, including Germany, France, and the United Kingdom, have witnessed a significant rise in the use of diagnostic kits, with many hospitals adopting advanced technologies. The European Medicines Agency (EMA) plays a pivotal role in facilitating the approval of new diagnostic products, which supports bladder cancer detective kit market expansion. However, varying reimbursement policies across European countries may present challenges.
The Middle East and Africa region is in the early stages of market development but is experiencing gradual improvements. In countries like the United Arab Emirates and Saudi Arabia, rising healthcare investments and increasing awareness of cancer detection contribute to market growth. However, limited access to advanced diagnostic technologies in some areas, along with high treatment costs, slows market expansion. In Africa, the focus on healthcare infrastructure improvement and public health campaigns has led to rising awareness of bladder cancer, further stimulating demand for diagnostic kits.
Latin America is witnessing steady growth in the bladder cancer detective kit market, with Brazil and Mexico leading the demand for diagnostic solutions. Increased healthcare access, higher disposable income, and rising awareness about cancer prevention contribute to market development. Public health campaigns and the adoption of advanced medical technologies in urban areas drive market expansion. However, disparities in healthcare access in rural areas and limited insurance coverage may hinder full market potential.
Top Key Players & Market Share Insights:
The Global Bladder Cancer Detective Kit Market is highly competitive with major players providing products and services to the national and international markets. Key players are adopting several strategies in research and development (R&D), product innovation, and end-user launches to hold a strong position in the Global Bladder Cancer Detective Kit Market. Key players in the Bladder Cancer Detective Kit industry include-
- Roche Diagnostics (Switzerland)
- Abbott Laboratories (United States)
- Becton, Dickinson and Company (United States)
- Medtronic PLC (Ireland)
- Sysmex Corporation (Japan)
- Siemens Healthineers (Germany)
- Thermo Fisher Scientific (United States)
- Danaher Corporation (United States)
- Hoffmann-La Roche (Switzerland)
- BioMerieux SA (France)
Recent Industry Developments :
Product Launches:
- In February 2025, Cxbladder offered a suite of non-invasive, urine-based tests designed to help rule out urothelial bladder cancer in patients experiencing hematuria and to monitor for recurrent disease in those previously treated for non-muscle invasive bladder cancer.
Bladder Cancer Detective Kit Market Report Insights:
| Report Attributes | Report Details |
| Study Timeline | 2019-2032 |
| Market Size in 2032 | USD 4,786.97 Million |
| CAGR (2025-2032) | 11.1% |
| By Product Type |
|
| By Technology |
|
| By End-User |
|
| By Distribution Channel |
|
| By Region |
|
| Key Players |
|
| North America | U.S. Canada Mexico |
| Europe | U.K. Germany France Spain Italy Russia Benelux Rest of Europe |
| APAC | China South Korea Japan India Australia ASEAN Rest of Asia-Pacific |
| Middle East and Africa | GCC Turkey South Africa Rest of MEA |
| LATAM | Brazil Argentina Chile Rest of LATAM |
| Report Coverage |
|
Key Questions Answered in the Report
How big is the Bladder Cancer Detective Kit Market? +
In 2024, the Bladder Cancer Detective Kit Market was USD 2,062.33 million.
What will be the potential market valuation for the Bladder Cancer Detective Kit Market by 2032? +
In 2032, the market size of Bladder Cancer Detective Kit Market is expected to reach USD 4,786.97 million.
What are the segments covered in the Bladder Cancer Detective Kit Market report? +
The product type, technology, distribution channel, and end-user are the segments covered in this report.
Who are the major players in the Bladder Cancer Detective Kit Market? +
Roche Diagnostics (Switzerland), Abbott Laboratories (United States), Siemens Healthineers (Germany), Thermo Fisher Scientific (United States), Danaher Corporation (United States), Hoffmann-La Roche (Switzerland), BioMerieux SA (France), Becton, Dickinson and Company (United States), Medtronic PLC (Ireland), Sysmex Corporation (Japan) are the major players in the Bladder Cancer Detective Kit market.