- Summary
- Table Of Content
- Methodology
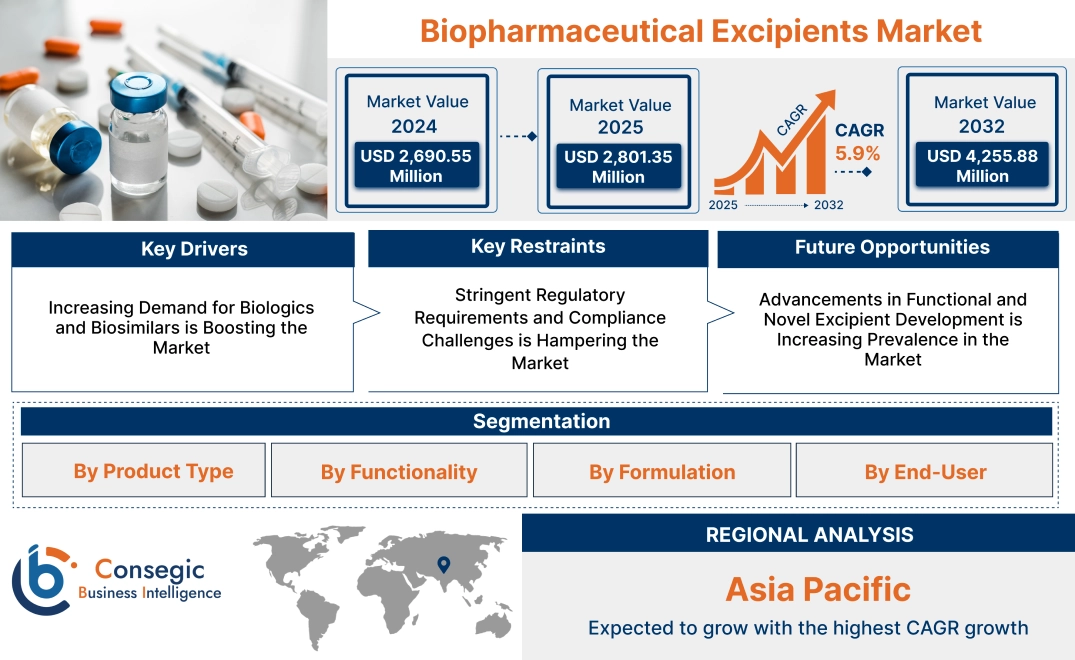
Biopharmaceutical Excipients Market Size:
Biopharmaceutical Excipients Market size is estimated to reach over USD 4,255.88 Million by 2032 from a value of USD 2,690.55 Million in 2024 and is projected to grow by USD 2,801.35 Million in 2025, growing at a CAGR of 5.9% from 2025 to 2032.
Biopharmaceutical Excipients Market Scope & Overview:
The biopharmaceutical excipients focus on inactive substances used in the formulation of biopharmaceutical products to enhance stability, bioavailability, and overall product performance. These excipients play a critical role in drug delivery, preservation, and controlled release of active pharmaceutical ingredients (APIs). Key categories include polymers, sugars, alcohols, amino acids, surfactants, and organic chemicals tailored to support biologics, vaccines, and gene therapies.
Key characteristics of biopharmaceutical excipients include high biocompatibility, chemical stability, and compatibility with sensitive biologic molecules. The benefits include improved drug efficacy, extended shelf life, and enhanced patient safety.
Applications span monoclonal antibodies, recombinant proteins, vaccines, and cell and gene therapies. End-users include biopharmaceutical companies, contract manufacturing organizations (CMOs), and research institutions, driven by the rising demand for biologics, advancements in drug formulation technologies, and growing investment in personalized medicine and innovative therapies.
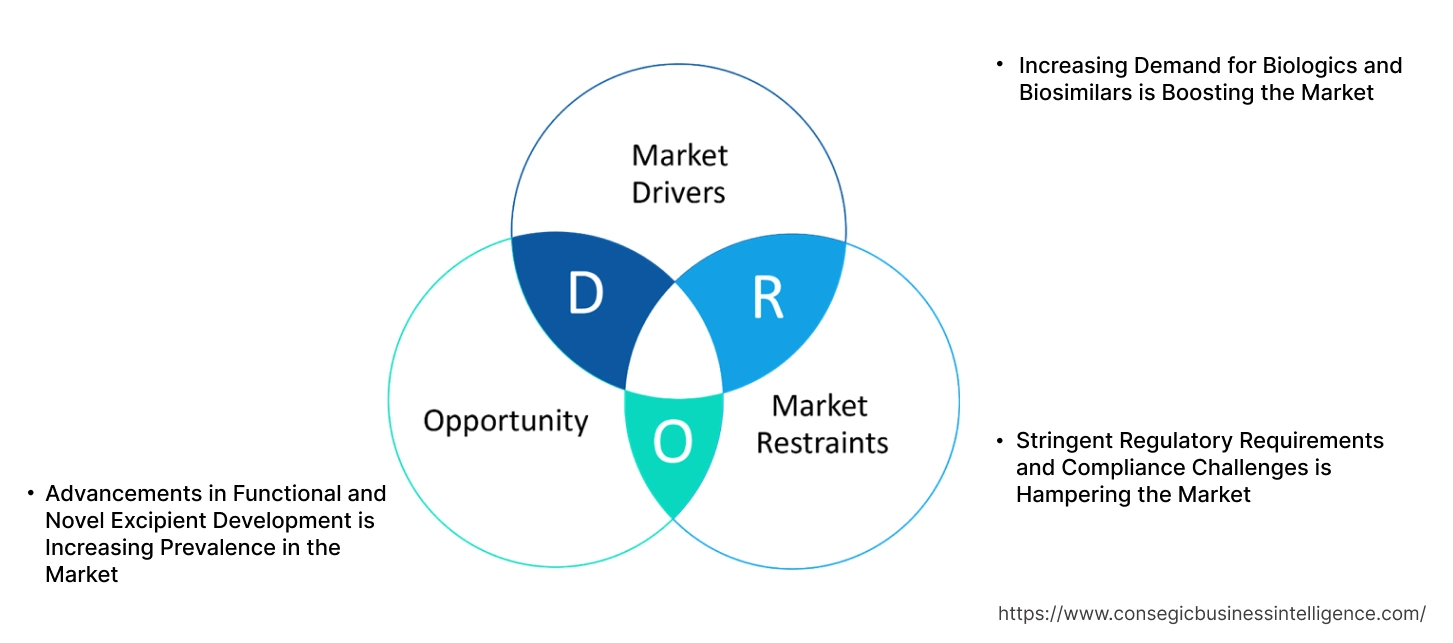
Key Drivers:
Increasing Demand for Biologics and Biosimilars is Boosting the Market
The rising dominance for biologics and biosimilars is a primary driver for the market. Biologics, including monoclonal antibodies, vaccines, recombinant proteins, and gene therapies, have transformed the treatment landscape for chronic diseases such as cancer, autoimmune disorders, and diabetes. As these therapies are complex and sensitive to environmental conditions, they require specialized excipients to enhance their stability, solubility, and bioavailability. With the expiration of patents for many blockbuster biologics, biosimilars are rapidly entering the market, further increasing the development for innovative excipient solutions that ensure the safety, efficacy, and shelf-life of these products. The surge in global biologics production is creating substantial growth biopharmaceutical excipients market opportunities for excipient manufacturers to develop customized solutions tailored to these complex therapies.
Key Restraints:
Stringent Regulatory Requirements and Compliance Challenges is Hampering the Market
The biopharmaceutical excipients market faces significant challenges due to stringent regulatory requirements and complex compliance standards. Regulatory authorities such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have strict guidelines for excipient quality, safety, and functionality. Excipients used in biopharmaceutical formulations must undergo rigorous testing and documentation to meet regulatory approval, which increases development costs and extends time-to-market. Additionally, variations in regulatory standards across regions create further compliance complexities for manufacturers operating globally. These regulatory hurdles can delay product launches and limit the introduction of novel excipient technologies, particularly for small and medium-sized companies with limited resources.
Future Opportunities:
Advancements in Functional and Novel Excipient Development is Increasing Prevalence in the Market
The development of functional and novel excipients presents a significant opportunity for market expansion. There is a growing focus on excipients that can enhance the delivery of biologics and biosimilars, including lipid-based nanoparticles (LNPs), polymeric carriers, and amino acid-based excipients. For instance, lipid nanoparticles have been pivotal in the successful delivery of mRNA vaccines, demonstrating the potential of innovative excipient technologies in improving drug stability and delivery efficiency. Furthermore, the demand for multi-functional excipients that combine multiple roles—such as stabilizing agents, solubilizers, and drug release modifiers—is increasing to streamline formulation processes and improve drug efficacy. Companies investing in research and development to create excipients that address solubility, stability, and targeted delivery challenges in biologics are well-positioned to capitalize on this market trends. Additionally, biodegradable and biocompatible excipients are gaining traction as sustainability becomes a greater priority in pharmaceutical manufacturing.
These dynamics underscore the growing importance of biopharmaceutical excipients in supporting the development and delivery of complex biologics and biosimilars. While regulatory challenges remain a barrier, advancements in functional and novel excipient technologies present promising biopharmaceutical excipients market opportunities for market growth, enabling safer, more effective, and sustainable therapeutic solutions.
Biopharmaceutical Excipients Market Segmental Analysis :
By Type:
Based on type, the market is segmented into organic excipients and inorganic excipients.
The organic excipients segment accounted for the largest revenue in biopharmaceutical excipients market share in 2024.
- Organic excipients, including polymers, sugars, alcohols, and proteins, are widely used due to their biocompatibility and safety profile.
- Increasing opportunities for complex biologics and large molecule drugs drives the need for advanced organic excipients.
- Polymers, particularly polyethylene glycol (PEG) and polysorbates, are extensively used for controlled drug release and drug stability.
- The growing trends toward natural and plant-based excipients is further boosting the organic excipients segment.
The inorganic excipients segment is anticipated to register the fastest CAGR during the forecast period.
- Inorganic excipients such as calcium phosphates, magnesium stearate, and silicon dioxide are essential for enhancing drug stability and flow properties.
- Increasing use in solid dosage forms for their compressibility and anti-caking properties is driving biopharmaceutical excipients market demand.
- Growing adoption of inorganic excipients in combination with organic ones for specialized formulations supports growth.
- Ongoing innovations in inorganic excipient functionality for biologics and injectable drugs are expected to propel market expansion.
By Functionality:
Based on functionality, the market is segmented into fillers & diluents, binders, coating agents, disintegrants, preservatives, solubilizers & stabilizers, and others.
The fillers & diluents segment accounted for the largest revenue in biopharmaceutical excipients market share in 2024.
- Fillers and diluents are essential in achieving the desired bulk and stability of drug formulations, especially in solid oral dosage forms.
- Increasing production of tablets and capsules globally drives consistent development for fillers and diluents.
- Widely used substances include lactose, microcrystalline cellulose, and calcium phosphate, supporting biopharmaceutical excipients market trends in this segment.
- Growing advancement for patient-friendly dosage forms, such as chewable and dispersible tablets, fuels advancement.
The solubilizers & stabilizers segment is anticipated to register the fastest CAGR during the forecast period.
- Solubilizers and stabilizers are critical for enhancing the bioavailability of poorly soluble drugs and biologics.
- Rising dominance for innovative drug delivery systems, including nanoparticles and liposomes, drives biopharmaceutical excipients market growth.
- Increasing use of advanced solubilizers like cyclodextrins and surfactants in complex biologics supports market expanding.
- Growing focus on improving drug stability and shelf life through excipient innovation is expected to propel this segment.
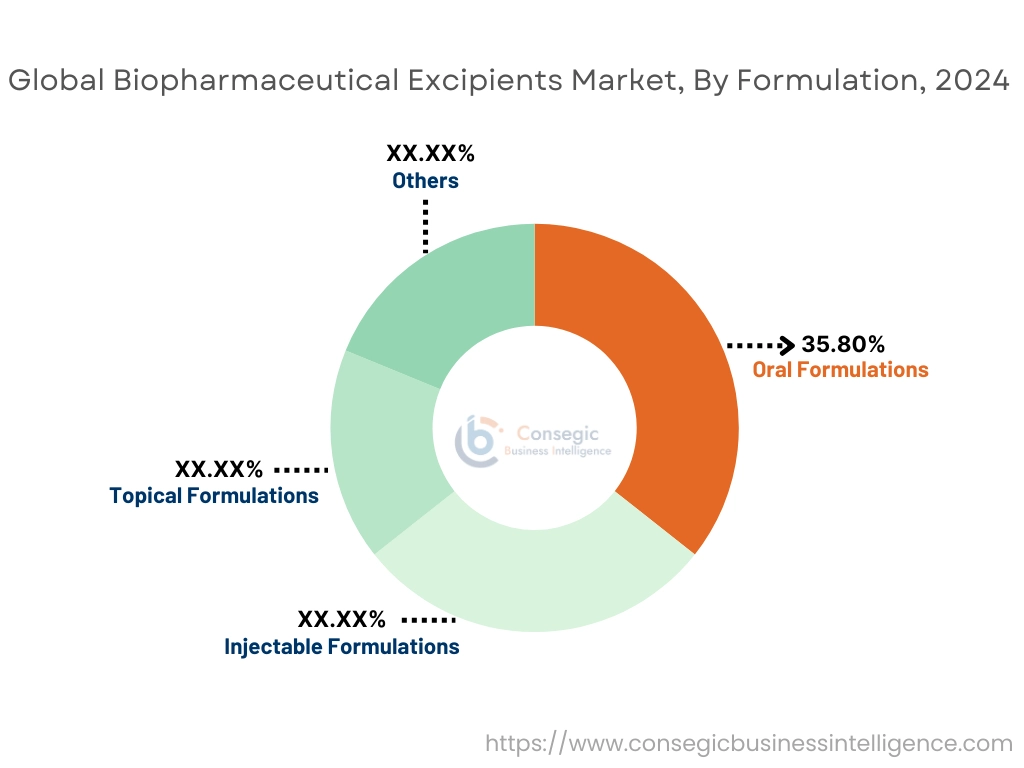
By Formulation:
Based on formulation, the market is segmented into oral formulations, injectable formulations, topical formulations, and others.
The oral formulations segment accounted for the largest revenue share of 35.80% in 2024.
- Oral formulations are the most widely used drug delivery route due to their convenience and patient compliance.
- High demand for tablets, capsules, and liquid forms drives consistent use of excipients in oral formulations.
- Growing geriatric population and increasing biopharmaceutical excipients market trends for orally disintegrating tablets (ODTs) support segment dominance.
- Expanding production of generic drugs and over-the-counter (OTC) medications fuels growth in this segment.
The injectable formulations segment is anticipated to register the fastest CAGR during the forecast period.
- Increasing advancement for biologics, vaccines, and biosimilars has significantly boosted the need for injectable formulations.
- Injectable drugs require specialized excipients for solubility, stability, and extended shelf life.
- Rising adoption of prefilled syringes and self-injectable devices is driving surge for innovative excipient solutions.
- Ongoing advancements in biologic drug development are expected to propel trends in this segment.
By End-User:
Based on end-user, the market is segmented into biopharmaceutical companies, contract research organizations (CROs), and research institutes.
The biopharmaceutical companies segment accounted for the largest revenue share in 2024.
- Biopharmaceutical companies are the primary consumers of excipients for large-scale drug development and production.
- Increasing R&D investments in biologics, biosimilars, and personalized medicines drive demand for specialized excipients.
- Growing focus on improving drug efficacy and safety through excipient innovation supports segment trends.
- Strategic partnerships between excipient manufacturers and biopharmaceutical companies are boosting product development.
The contract research organizations (CROs) segment is anticipated to register the fastest CAGR during the forecast period.
- CROs are increasingly relied upon for outsourced drug development and clinical trials, driving excipient development.
- Rising trends of pharmaceutical outsourcing to reduce costs and improve efficiency supports segment analysis.
- Growing surge for specialized excipients in clinical-stage biologics fuels adoption by CROs.
- Expansion of CRO services in emerging markets is expected to propel this segment's growth.
Regional Analysis:
The regions covered are North America, Europe, Asia Pacific, the Middle East and Africa, and Latin America.
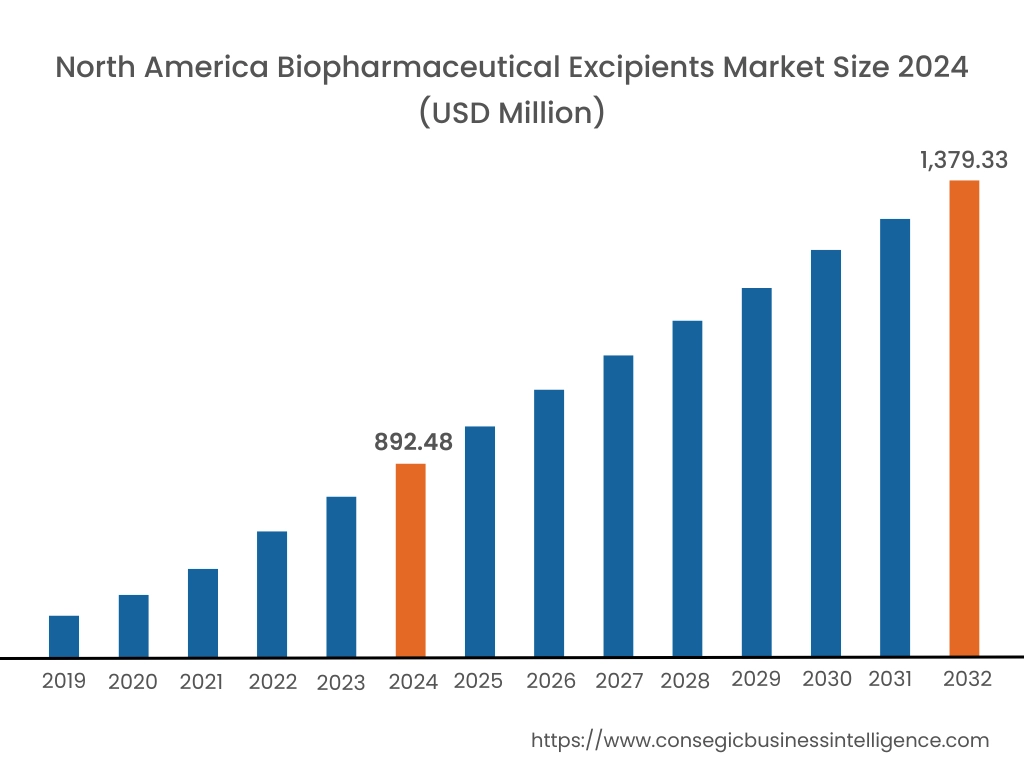
In 2024, North America was valued at USD 892.48 Million and is expected to reach USD 1,379.33 Million in 2032. In North America, the U.S. accounted for the highest share of 71.60% during the base year of 2024. North America holds a dominant share in the global biopharmaceutical excipients market, driven by the strong presence of leading pharmaceutical and biopharmaceutical companies, advanced drug manufacturing technologies, and high investments in research and development. The U.S. leads the region due to its robust regulatory framework that promotes the development of innovative drug formulations, including excipient technologies. The growing demand for biologics and biosimilars further fuels the need for high-quality excipients. As per the analysis, Canada contributes with its expanding biopharmaceutical sector and increasing focus on drug development. However, stringent FDA regulations on excipient approval may slow the introduction of novel excipients.
In Asia Pacific, the market is experiencing the fastest growth with a CAGR of 6.3% over the forecast period. Asia-Pacific is the fastest-growing region in the biopharmaceutical excipients market, fueled by rapid advancement in pharmaceutical manufacturing, increasing investments in biologics production, and improving regulatory frameworks in China, India, and Japan. As per the analysis, China dominates the region with rising progress for biologic drugs and government-led initiatives to expand biopharmaceutical manufacturing capabilities. India’s expanding generic and biosimilar drug markets drive the market surge for cost-effective and functional excipients. Japan focuses on high-quality excipients for advanced drug delivery systems, leveraging its strong pharmaceutical research capabilities. However, quality concerns and inconsistent regulatory standards across countries may hinder the adoption of innovative excipients.
Europe is a prominent market for biopharmaceutical excipients, supported by a strong pharmaceutical industry, growing demand for innovative drug formulations, and favorable regulatory policies. Countries like Germany, France, and the UK are key contributors. As per the biopharmaceutical excipients market analysis, Germany leads with its advanced drug manufacturing infrastructure and focus on quality excipient production. France emphasizes investments in biopharmaceutical R&D and partnerships between excipient manufacturers and pharmaceutical companies. The UK drives market enlargement with its strong presence in biologics and personalized medicine. However, regulatory complexities surrounding excipient approvals may pose challenges for biopharmaceutical excipients market expansion.
The Middle East & Africa region is witnessing steady development in the market, driven by increasing investments in healthcare infrastructure and a growing pharmaceutical industry. Countries like Saudi Arabia and the UAE are focusing on expanding their pharmaceutical manufacturing capabilities, supported by government initiatives to reduce dependency on imports. In Africa, South Africa is emerging as a key market, driven by growing trends for affordable biologics and partnerships with global pharmaceutical companies. However, limited local production of excipients and regulatory challenges may restrict biopharmaceutical excipients market growth in certain parts of the region.
Latin America is an emerging market for biopharmaceutical excipients, with Brazil and Mexico leading the region. Brazil’s growing pharmaceutical industry and rising dominance for biosimilars drive the need for innovative excipients to support drug formulation. The regional biopharmaceutical excipients market analysis, Mexico focuses on strengthening its biopharmaceutical manufacturing sector through public-private partnerships and investments in drug development. The region also benefits from increasing collaborations with international pharmaceutical companies. However, economic instability and inconsistent regulatory frameworks may pose challenges to market expansion in smaller economies.
Top Key Players & Market Share Insights:
The biopharmaceutical excipients market is highly competitive with major players providing products and services to the national and international markets. Key players are adopting several strategies in research and development (R&D), product innovation, and end-user launches to hold a strong position in the global biopharmaceutical excipients market. Key players in the biopharmaceutical excipients industry include -
- BASF SE (Germany)
- Ashland Global Holdings Inc. (United States)
- Colorcon Inc. (United States)
- Avantor, Inc. (United States)
- Shin-Etsu Chemical Co., Ltd. (Japan)
- Evonik Industries AG (Germany)
- Croda International Plc (United Kingdom)
- FMC Corporation (United States)
- Roquette Frères (France)
- Lubrizol Corporation (United States)
Biopharmaceutical Excipients Market Report Insights:
| Report Attributes | Report Details |
| Study Timeline | 2019-2032 |
| Market Size in 2032 | USD 4,255.88 Million |
| CAGR (2025-2032) | 5.9% |
| By Product Type |
|
| By Functionality |
|
| By Formulation |
|
| By End-User |
|
| By Region |
|
| Key Players |
|
| North America | U.S. Canada Mexico |
| Europe | U.K. Germany France Spain Italy Russia Benelux Rest of Europe |
| APAC | China South Korea Japan India Australia ASEAN Rest of Asia-Pacific |
| Middle East and Africa | GCC Turkey South Africa Rest of MEA |
| LATAM | Brazil Argentina Chile Rest of LATAM |
| Report Coverage |
|
Key Questions Answered in the Report
What is the market size and growth potential by 2032? +
Biopharmaceutical Excipients Market size is estimated to reach over USD 4,255.88 Million by 2032 from a value of USD 2,690.55 Million in 2024 and is projected to grow by USD 2,801.35 Million in 2025, growing at a CAGR of 5.9% from 2025 to 2032.
What factors are driving market growth? +
Rising biologics production, growing biosimilars demand, advancements in excipient technologies, and increasing investments in personalized medicine are the key growth drivers.
What challenges are limiting market growth? +
Stringent regulatory requirements, high production costs, and complex compliance standards hinder market expansion, especially for small manufacturers.
Which segment holds the largest market share? +
The organic excipients segment dominates due to its biocompatibility and widespread use in stabilizing biologics and biosimilars.
Which region leads the market? +
North America leads the market due to advanced pharmaceutical infrastructure, with Asia Pacific showing the fastest growth driven by expanding biopharma industries.