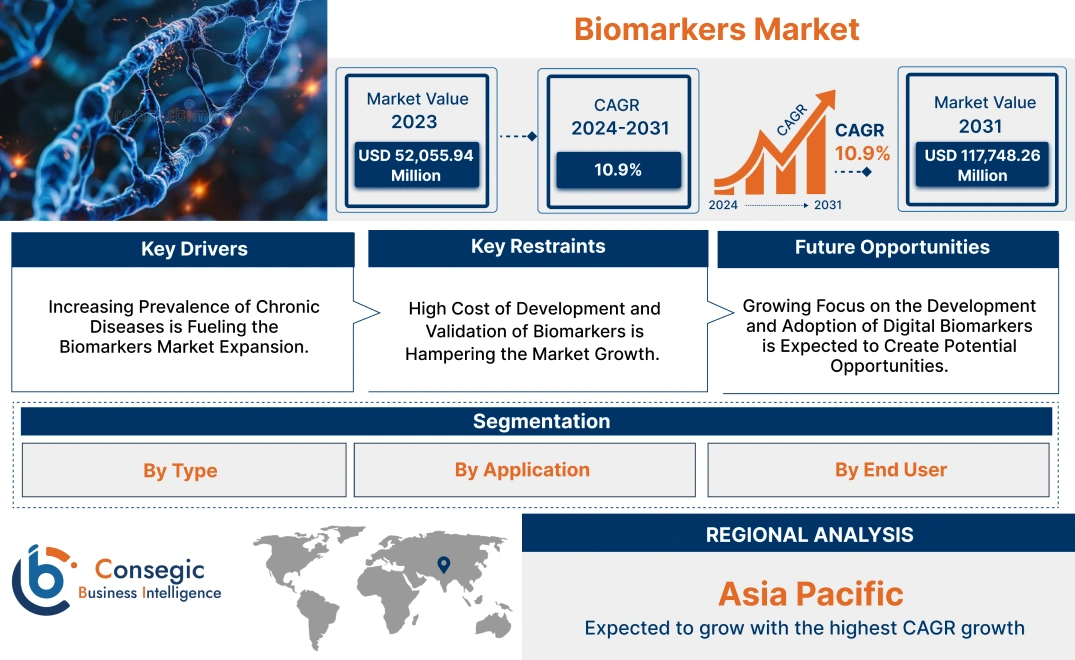
Biomarkers Market Size:
Biomarkers Market size is growing with a CAGR of 10.9% during the forecast period (2024-2031), and the market is projected to be valued at USD 117,748.26 Million by 2031 from USD 52,055.94 Million in 2023.
Biomarkers Market Scope & Overview:
A biomarker also known as a biological marker is an indicator that represents a sign of a normal or abnormal biological state or response to a therapeutic intervention. It's like a biological signpost that can indicate the presence of a disease, how severe it is, or how well a treatment is working. It is a biological molecule that is measured to predict, diagnose, and monitor health conditions. Quantifying and evaluating biomarkers serve as an indicator of normal biological processes, pathogenic processes, and pharmacological responses to a therapeutic intervention. They are classified as susceptible/risk, diagnostic, monitoring, prognostic, predictive, response, and safety among others. They provide disease prediction, diagnosis, and assessment of toxicity. Hence, they are widely utilized for several applications such as diagnosis, drug discovery & development, and precision among others.
How is AI Impacting the Biomarkers Market?
A biomarker also known as a biological marker is an indicator that represents a sign of a normal or abnormal biological state or response to a therapeutic intervention. It's like a biological signpost that can indicate the presence of a disease, how severe it is, or how well a treatment is working. It is a biological molecule that is measured to predict, diagnose, and monitor health conditions. Quantifying and evaluating biomarkers serve as an indicator of normal biological processes, pathogenic processes, and pharmacological responses to a therapeutic intervention. They are classified as susceptible/risk, diagnostic, monitoring, prognostic, predictive, response, and safety among others. They provide disease prediction, diagnosis, and assessment of toxicity. Hence, they are widely utilized for several applications such as diagnosis, drug discovery & development, and precision among others.
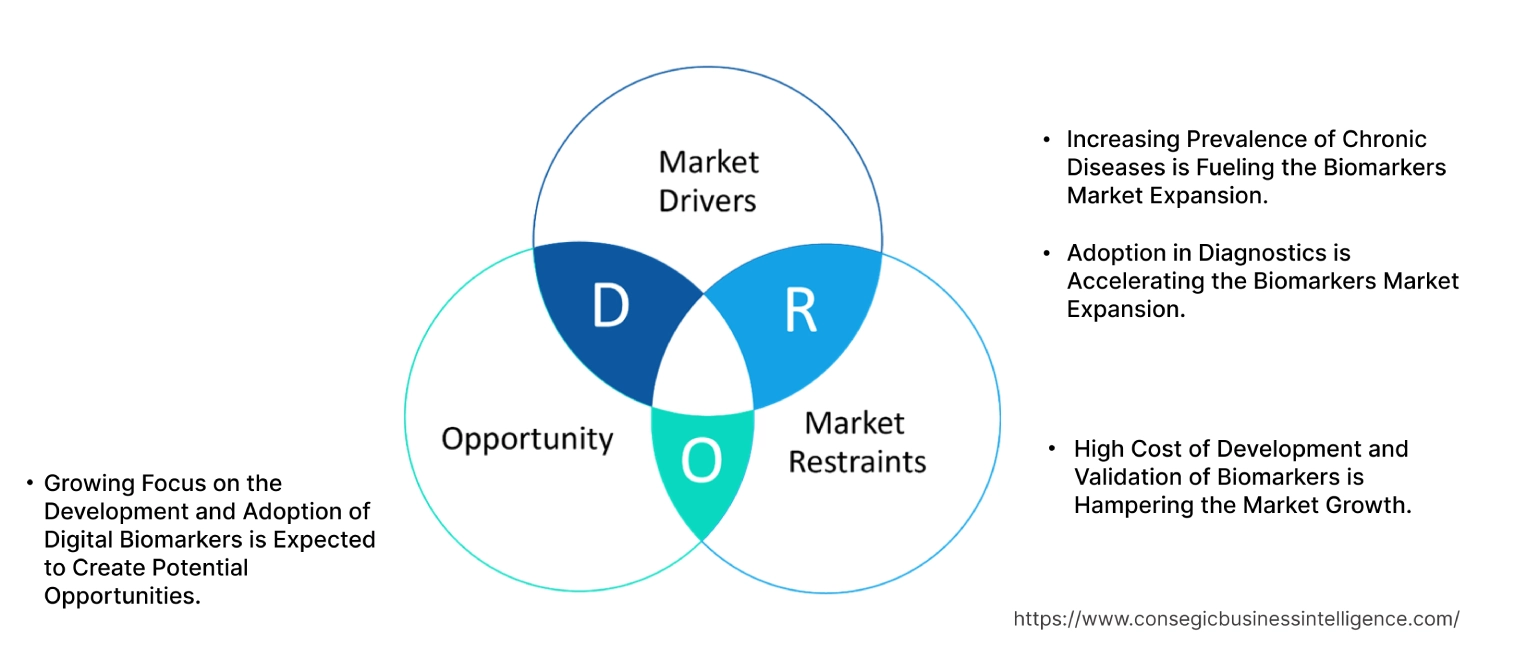
Biomarkers Market Dynamics - (DRO) :
Key Drivers:
Increasing Prevalence of Chronic Diseases is Fueling the Biomarkers Market Expansion.
The escalating prevalence of chronic diseases serves as one of the prominent factors in the biomarkers market growth. As individual age and lifestyles change, the incidence of conditions such as cancer, diabetes, cardiovascular diseases, and neurodegenerative disorders is rising.
For instance,
- According to the analysis provided by the World Health Organization in February 2022, cancer is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020, or nearly one in six deaths. The most common cancers are breast, lung, colon, rectum, and prostate cancers. Cancer-causing infections, such as human papillomavirus (HPV) and hepatitis, are responsible for approximately 30% of cancer cases in low- and lower-middle-income countries.
Biomarkers offer a suitable tool for early disease detection, enabling timely intervention and improved patient outcomes. By identifying specific molecular markers associated with these diseases, healthcare providers to diagnose conditions at earlier stages, when treatment is often more effective.
Additionally, healthcare providers are increasingly focusing on early diagnosis and treatment to prevent complications and improve patient outcomes. Thus, the rising prevalence of chronic diseases fuels a significant rise in the global biomarkers market trends.
Adoption in Diagnostics is Accelerating the Biomarkers Market Expansion.
Biomarker tests, which measure specific biological molecules in blood, urine, or tissue samples, provide valuable insights into a patient's health status and aid in early disease detection and accurate diagnosis. As healthcare providers seek more precise and effective diagnostic solutions, the demand for biological marker tests is growing.
These tests help identify individuals at risk of developing certain diseases, distinguish between different conditions with similar symptoms, and monitor disease progression. In cancer diagnosis, they help identify tumors at early stages. In cardiovascular disease, they assess the risk of heart attack and stroke. Owing to the major role played by biomarkers in diagnostics several tests are being developed.
- For instance, in July 2023, Quanterix Corporation announced the launch of the LucentAD biomarker blood test to aid physician diagnosis of Alzheimer’s disease (AD) in patients. The LucentAD test, available to healthcare providers as an aid in conjunction with other diagnostic tools, provides clinicians with a simplified process to quickly assess the likelihood of a patient having amyloid pathology consistent with AD. This supports healthcare providers in diagnosing, determining appropriate follow-up, and treatment planning for a suspected Alzheimer’s patient.
The growth in the availability of point-of-care testing devices is making this testing more accessible, particularly in remote and underserved areas. By supporting healthcare providers with accurate and timely diagnostic information, biological marker tests are advancing healthcare.
Key Restraints :
High Cost of Development and Validation of Biomarkers is Hampering the Market Growth.
The complex process of identifying, validating, and commercializing biomarkers requires substantial investments in research and development. The identification of novel biological markers often involves extensive research and development efforts. This includes large-scale studies, advanced analytical techniques, and bioinformatics tools to analyze vast amounts of data. Many biological marker assays rely on sophisticated analytical techniques, such as mass spectrometry, flow cytometry, and next-generation sequencing, which becomes expensive to acquire and operate. These techniques require highly skilled personnel to operate, interpret, and perform the analysis of the results, further increasing costs.
Additionally, the validation is a critical step to ensure their accuracy, reliability, and clinical utilization. Biomarker development and validation must adhere to strict regulatory guidelines, which involve time-consuming and expensive clinical trials and regulatory submissions. This process involves rigorous clinical trials and statistical analysis to establish the biological marker's performance characteristics. These studies are time-consuming and expensive, as they require large sample sizes &, specialized expertise, and rigorous quality control measures. Overall, the complexity of development and validation significantly increases the cost further hindering the biomarkers market expansion.
Future Opportunities :
Growing Focus on the Development and Adoption of Digital Biomarkers is Expected to Create Potential Opportunities.
Digital biomarkers are quantifiable, physiological, and behavioral measures. They are collected by means of digital devices that are portable, wearable, implantable, or digestible. This data is used to explain, analyze, and predict health-related outcomes.
Integrating these into clinical practice, allows healthcare providers to gain deeper insights into patient health, enabling earlier disease detection, more accurate diagnosis, and personalized treatment plans. This creates the demand for the development and adoption of these markers by pharmaceutical and biotechnology companies and healthcare providers.
- For instance, in November 2024, Neuron23 Inc., a clinical-stage biotechnology company focused on developing precision medicines for genetically defined neurological and immunological diseases, announced a collaboration with Roche to incorporate a navy digital biomarker developed by Roche Information Solutions, as the primary endpoint in the clinical trial in early-stage Parkinson’s disease.
Thus, as the field of digital healthcare continues to experience growth, the integration of digital biomarkers into clinical practice is expected to drive significant advancements in healthcare further creating biomarkers market opportunities.
Biomarkers Market Segmental Analysis :
By Type:
Based on type, the market is categorized into susceptibility/risk, diagnostic, monitoring, prognostic, predictive, safety, and others.
Trends in the Type:
- Increasing focus on identifying genetic variants associated with disease risk.
- Advances in liquid biopsy techniques for early cancer detection and monitoring.
- Real-time monitoring of biological marker levels to track disease progression and treatment response.
The safety biomarker segment accounted for the largest market share in 2023.
- Safety biomarkers indicate the likelihood, presence, or extent of toxicity as an adverse effect of exposure to a medical product. This biological marker is measured before and after exposure to a medical product.
- Safety biological marker plays a crucial role in ensuring the safety and efficacy of new drugs. Monitoring changes in specific biological markers allows researchers and clinicians to identify potential adverse effects and take steps to mitigate risks.
- These markers help detect early signs of toxicity, such as organ damage, and allow for timely intervention to prevent serious health consequences.
- By incorporating this type of marker into drug development and clinical trials, they help improve the safety of new therapies and reduce the risk of serious side effects.
- Regulatory agencies impose rigorous safety assessment guidelines for new drugs and medical devices driving the demand. Safety biological markers play a crucial role in these assessments, ensuring patient safety and regulatory compliance.
- Overall, analysis of safety biological markers ensures the development of safe and effective medical products, ultimately benefiting patient health, boosting the biomarkers market growth.
The diagnostic segment is expected to grow at the fastest CAGR over the forecast period.
- Diagnostic biomarkers are used to detect or confirm the presence of a disease. They play a crucial role in early detection, accurate diagnosis, and effective management of diseases.
- Additionally, they help distinguish between different diseases with similar symptoms, leading to more accurate diagnoses. Biological markers such as prostate-specific antigen (PSA) are used for prostate cancer diagnosis.
- These markers, often proteins, genes, and metabolites among others, are detected in various bodily fluids, including blood, urine, and tissue samples. The development of novel diagnostic biomarkers is expected to create a potential rise in the segment trend.
- For instance, according to the research analysis provided in the Nature Journal in November 2024, it is stated that novel methylation markers provide non-invasive bladder cancer detection in urine. The excellent diagnostic potential of our methylation markers for non-invasive BC detection emphasizes their significance for future diagnostic strategies.
- Diagnostic biological marker is often used in conjunction with other diagnostic tests, such as imaging studies or clinical examinations.
- Thus, analysis of diagnostic biological markers provides more accurate and timely diagnoses that make them crucial components in the biomarkers industry.
By Application:
Based on application, the market is categorized into drug discovery & development, diagnostics, personalized medicine, and others.
Trends in the Application:
- Leveraging artificial intelligence and machine learning trends to accelerate drug discovery by analyzing vast datasets and identifying novel biological markers.
- Developing early-stage diagnostic tests for diseases such as cancer and Alzheimer's.
- Using biomarkers to guide personalized treatment plans, improving patient outcomes.
The diagnosis segment accounted for the largest market share of the biomarkers market share in the year 2023.
- Biomarkers serve a crucial role in the field of diagnostics, advancing the way diseases are detected and monitored. By identifying specific biological molecules associated with various health conditions, biological markers enable early disease detection, accurate diagnosis, and effective treatment.
- They are employed to diagnose several conditions such as cancer, neurological disorders, autoimmune diseases, and infectious diseases.
- By providing early warning signs and aiding in differential diagnosis, biological markers significantly improve patient outcomes and facilitate timely interventions. The growing focus on the development of testing products for diagnostics significantly contributes to the segment growth.
- For instance, in August 2024, Hitachi High-Tech Corporation and Gencurix, Inc. entered a strategic partnership in the field of cancer molecular diagnostics. The Partnership aims to develop a testing service for cancer molecular diagnostics by combining Hitachi High-Tech's core expertise in R&D and manufacturing of in vitro diagnostic products and digital technology, and Gencurix's technology and experience in biological markers.
- Thus, the role of biological markers in diagnostics is expanding, leading to more precise and effective healthcare, proliferating the biomarkers market demand.
The drug discovery & development segment is expected to grow at the fastest CAGR over the forecast period.
- Biomarkers play a crucial role in accelerating drug discovery and development processes. They help identify potential drug targets, assess drug efficacy, and predict adverse drug reactions.
- Understanding the molecular mechanisms of disease allows researchers to develop targeted therapies that are more effective and have fewer side effects.
- They are also used to identify patient populations that are most likely to benefit from specific treatments, optimizing clinical trial design and reducing development costs.
- Additionally, biomarkers are used to assess the safety and efficacy of new drugs. By monitoring changes in biological marker levels, researchers are able to evaluate the impact of a drug on a specific biological pathway and identify potential adverse effects early in the development process. This enables researchers to make informed decisions about drug development and reduce the risk of failure in clinical trials.
- Thus, leveraging biological markers allows drug discovery, optimizes clinical trials, and ultimately develops more effective and safer therapies.
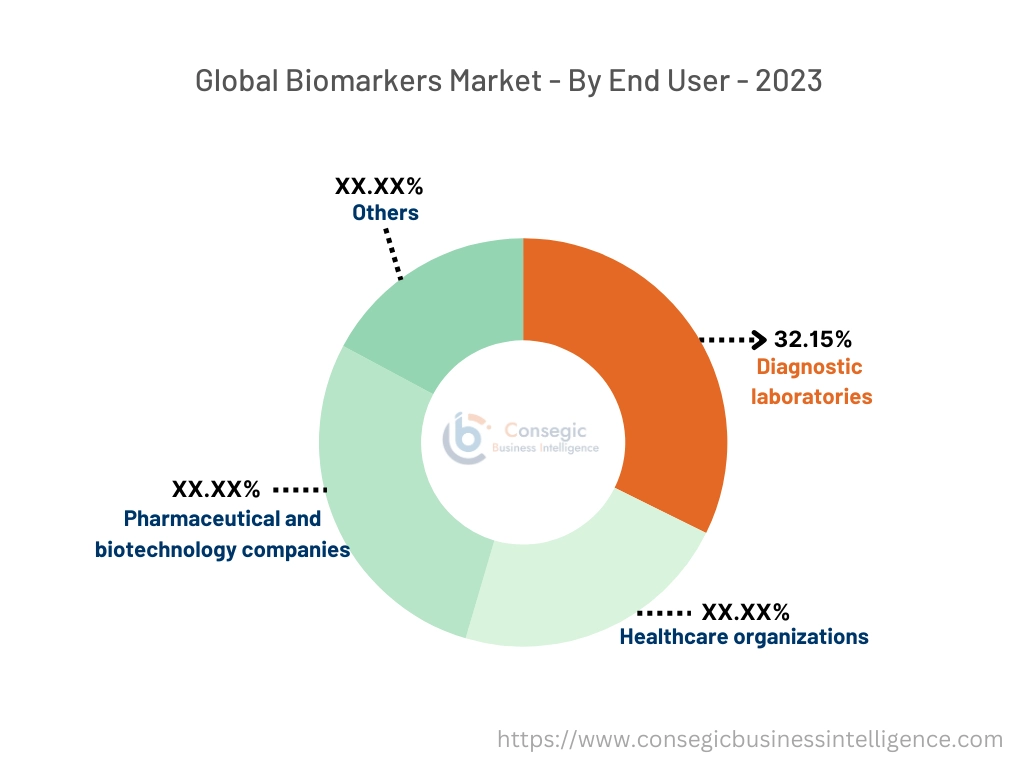
By End-User:
Based on end users, the market is categorized into pharmaceutical and biotechnology companies, healthcare organizations, diagnostic laboratories, and others.
Trends in the End User:
- Leveraging biological markers to develop targeted therapies and personalized medicine approaches is a trend positively impacting demand from end users.
- Increasing the availability of point-of-care biomarker tests is a trend supporting demand from end users.
The diagnostic laboratories segment accounted for the largest market share of 32.15% in the year 2023 and is also expected to grow at the fastest CAGR over the forecast period.
- Diagnostic laboratories play a crucial role in the biomarker market by performing biomarker tests. These laboratories use advanced technologies to measure biological marker levels in different samples such as blood, urine, or tissue samples.
- The results of these tests provide valuable information for healthcare providers, helping them to diagnose diseases, monitor disease progression, and assess treatment response.
- Diagnostic laboratories also play a key role in research and development, collaborating with pharmaceutical companies and academic institutions to identify and validate new biological markers. Additionally, diagnostic laboratories serve a crucial role in developing biological marker
- For instance, in November 2024, Augurex Life Sciences Corp. announced an agreement with Quest Diagnostics, under which Quest will validate and offer a lab-developed test based on the Augurex 14-3-3η (eta) biomarker in the United States. Quest intends to begin offering the test service to physicians from its advanced laboratory by December 2, 2024.
- Overall, by developing and performing biological marker tests, diagnostic laboratories play a vital role in advancing healthcare, enabling earlier diagnosis, improved treatment, and better patient outcomes making it a dominant end user in the biomarkers market trend.
Regional Analysis:
The regional segment includes North America, Europe, Asia Pacific, the Middle East and Africa, and Latin America.
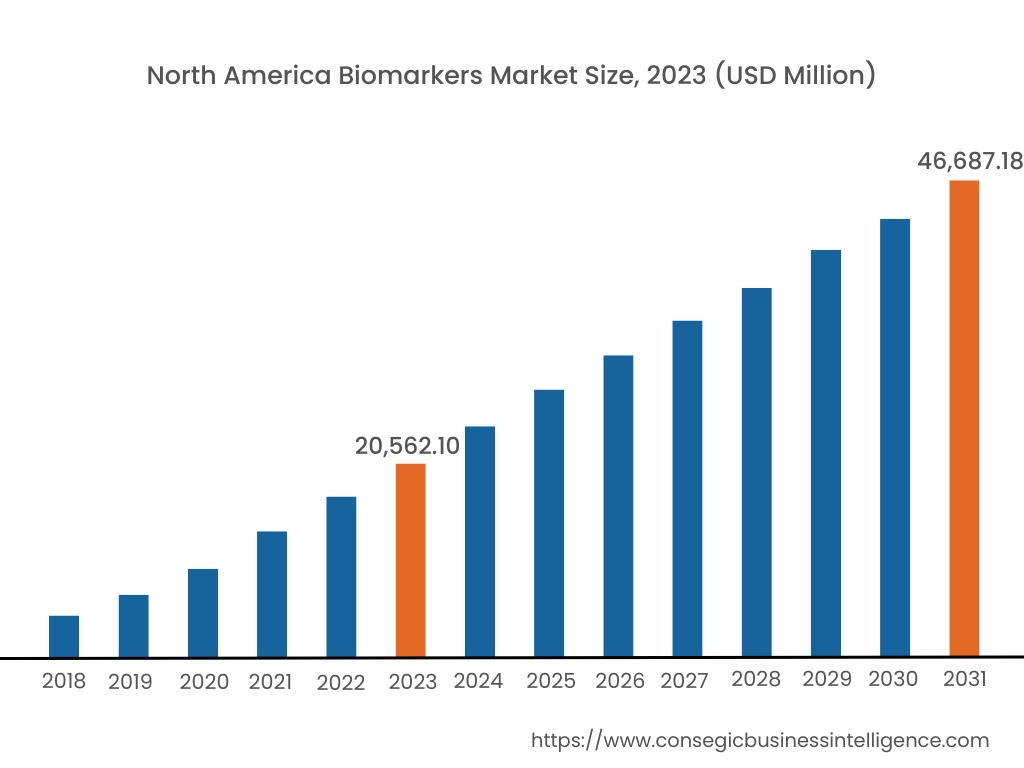
In 2023, North America accounted for the highest market share at 39.50% and was valued at USD 20,562.10 Million and is expected to reach USD 46,687.18 Million in 2031. In North America, the U.S. accounted for the highest market share of 66.50% during the base year of 2023.
The North American region is a significant player in the global market, driven by factors such as the advanced healthcare industry, robust regulatory frameworks, and a strong focus on research and development. The United States, in particular, holds major biomarkers market share, with a well-established healthcare system and a large patient population. The region boasts a strong presence of pharmaceutical and biotechnology companies, academic institutions, and diagnostic laboratories, all of which contribute to the market. Additionally, the efforts taken by regulatory bodies in the region to ensure the safety associated with biomarker tests are also supporting the biomarkers market analysis.
- For instance, in June 2023, the S. Food and Drug Administration introduced a new voluntary pilot program designed to mitigate risks associated with laboratory-developed tests (LDTs) used for cancer biomarker identification. This initiative aims to enhance the transparency and reliability of these tests, particularly those used to select specific oncology drug treatments. By providing performance recommendations, the FDA seeks to improve patient care and ensure the accuracy of diagnostic results.
The combination of the aforementioned factors and trends is driving substantial growth.
The Asia-Pacific is experiencing the fastest growth with a CAGR of 11.4% over the forecast period. This surge is attributed to factors such as the increasing prevalence of chronic diseases. Countries like China, India, Japan, and South Korea are experiencing a surge in demand for advanced healthcare solutions, including biomarker-based diagnostics. Additionally, increasing healthcare expenditure and a growing aging population also support the biomarker market trend across the region. The region's large population and high prevalence of chronic diseases, such as diabetes, cancer, and cardiovascular diseases, create significant market growth. Additionally, the growing number of pharmaceutical and biotechnology companies in Asia-Pacific is fueling the demand for biological markers in drug discovery and development.
Europe is a significant player in the market, driven by factors such as a strong healthcare industry, a focus on innovation, and stringent regulatory frameworks. The region presents well-established healthcare systems and a robust research ecosystem. This environment fosters the development and adoption of advanced biomarker technologies. The region's emphasis on personalized medicine and precision healthcare has further fueled the market. Additionally, the European Union's regulatory framework provides a clear regulatory pathway for biomarker-based diagnostic tests, ensuring their safety and efficacy. Furthermore, the region's strong academic institutions and research centers are actively engaged in biological marker research, leading to the discovery of novel biological markers and the development of innovative diagnostic tools.
The Middle East and Africa (MEA) region is an emerging market, driven by factors such as increasing healthcare expenditure, growing awareness of chronic diseases, and the adoption of advanced medical technologies. Many countries in the MEA region are investing in developing their healthcare, leading to increased requirements for advanced diagnostic solutions such as the identification of biomarkers. Additionally, the establishment of research centers and collaborations with international partners is contributing to the development of biomarker-based diagnostic and therapeutic solutions. As healthcare infrastructure improves and awareness of biological markers increases, it creates a favorable environment for the trajectory of the biomarkers market opportunities in the MEA region.
Latin America is an emerging region in the market. The confluence of the increasing prevalence of chronic diseases, increasing healthcare expenditure, and growing awareness of preventive healthcare are driving the significant rise of the market in Latin America. There is a growing demand for accurate and reliable diagnostic tools, particularly in areas with limited access to specialized healthcare. The increasing adoption of personalized medicine approaches, which rely heavily on biological markers, is further fueling the biomarkers market analysis in Latin America. These factors collectively present a promising factor for diagnostics laboratories, healthcare providers, and pharmaceutical companies manufacturers to develop and deliver innovative solutions such as the use of biomarkers in Latin America.
Top Key Players & Market Share Insights:
The biomarkers market is highly competitive with major players providing products to the national and international markets. Key players are adopting several strategies in research and development (R&D) and product innovation to hold a strong position in the global biomarkers market. Key players in the biomarkers industry include-
- Hoffmann-La Roche Ltd (Switzerland)
- Quanterix (U.S.)
- Agilent Technologies, Inc. (U.S.)
- EKF Diagnostics Holdings plc. (UK)
- PerkinElmer Inc. (U.S.)
- Illumina, Inc. (U.S.)
- Bruker (U.S.)
- Quest Diagnostics Incorporated (U.S.)
- Epigenomics AG (Germany)
- Thermo Fisher Scientific Inc. (U.S.)
Recent Industry Developments :
New Launch:
- In October 2024, Lucent Diagnostics, a brand of Quanterix Corporation introduced LucentAD Complete, a new multi-marker blood test designed to help detect Alzheimer’s Disease in a broader range of patients.
Partnership:
- In November 2024, Neuron23 Inc. announced a collaboration with Roche to incorporate a digital biomarker developed by Roche Information Solutions, as the primary endpoint in the clinical trial in early-stage Parkinson’s disease.
- In November 2024, Augurex Life Sciences Corp. announced an agreement with Quest Diagnostics, under which Quest will validate and offer a lab-developed test based on the Augurex 14-3-3η (eta) biomarker in the United States. Quest intends to begin offering the test service to physicians from its advanced laboratory by December 2, 2024.
- In August 2024, Hitachi High-Tech Corporation and Gencurix, Inc. entered a strategic partnership in the field of cancer molecular diagnostics. The Partnership aims to develop a testing service for cancer molecular diagnostics by combining Hitachi High-Tech's core expertise in R&D and manufacturing of in vitro diagnostic products and digital technology, and Gencurix's technology and experience in biomarkers.
- In February 2024, Biofourmis, announced four new agreements with the top 20 pharmaceutical companies. The new engagements focus on oncology therapies. Centered on developing digital biomarkers and safety monitoring algorithms, with a specific focus on cytokine release syndrome (CRS) detection, the oncology programs leverage cutting-edge data science and technology to enhance remote data collection, patient monitoring, and safety.
Biomarkers Market Report Insights :
| Report Attributes | Report Details |
| Study Timeline | 2018-2031 |
| Market Size in 2031 | USD 117,748.26 Million |
| CAGR (2024-2031) | 10.9% |
| By Type |
|
| By Application |
|
| By End-User |
|
| By Region |
|
| Key Players |
|
| North America | U.S. Canada Mexico |
| Europe | U.K. Germany France Spain Italy Russia Benelux Rest of Europe |
| APAC | China South Korea Japan India Australia ASEAN Rest of Asia-Pacific |
| Middle East and Africa | GCC Turkey South Africa Rest of MEA |
| LATAM | Brazil Argentina Chile Rest of LATAM |
| Report Coverage |
|
Key Questions Answered in the Report
How big is the Biomarkers market? +
In 2023, the Biomarkers market is USD 52,055.94 Million.
Which is the fastest-growing region in the Biomarkers market? +
Asia Pacific is the fastest-growing region in the Biomarkers market.
What specific segmentation details are covered in the Biomarkers market? +
Type, Application, and End-user segmentation details are covered in the Biomarkers market
Who are the major players in the Biomarkers market? +
F. Hoffmann-La Roche Ltd (Switzerland), Quanterix (U.S.), and Illumina, Inc. (U.S.) are some of the major players in the market.