- Summary
- Table Of Content
- Methodology
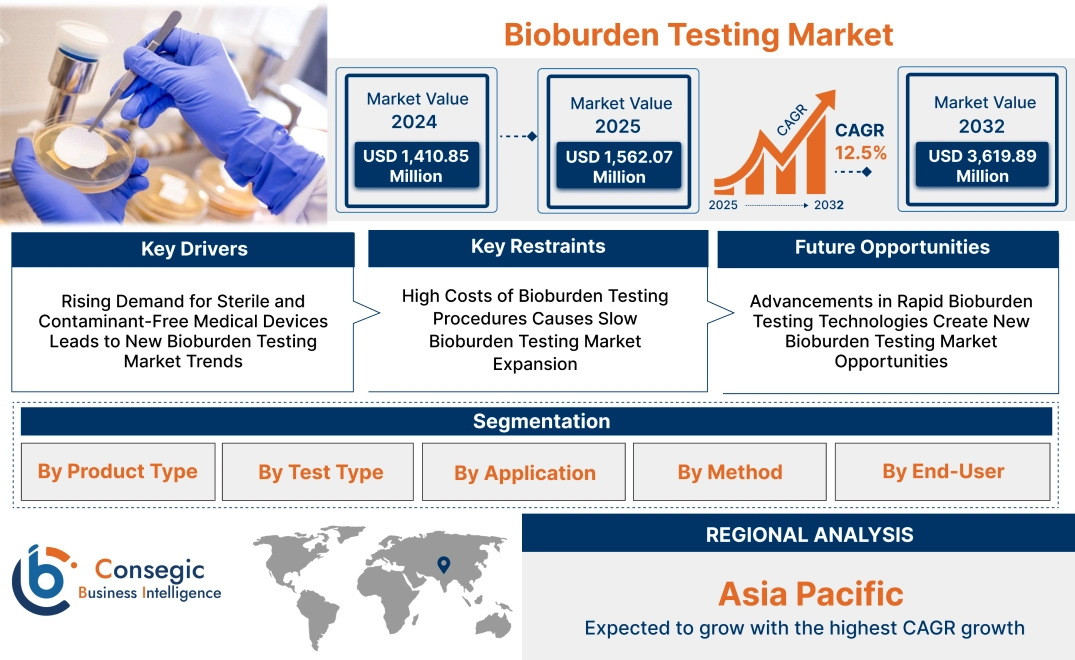
Bioburden Testing Market Size:
Bioburden Testing Market size is estimated to reach over USD 3,619.89 Million by 2032 from a value of USD 1,410.85 Million in 2024 and is projected to grow by USD 1,562.07 Million in 2025, growing at a CAGR of 12.5% from 2025 to 2032.
Bioburden Testing Market Scope & Overview:
Bioburden testing is a quantitative process that measures microbial contamination levels in products or materials during production and before sterilization. It is a critical component in quality control, ensuring safety and compliance with regulatory standards. This testing is commonly used in industries where microbial contamination poses significant risks, including pharmaceuticals, medical devices, biotechnology, and food and beverages. This testing exhibits properties like precision, reliability, and adaptability to various sample types. It employs methods such as membrane filtration, direct plating, and automated technologies to detect and enumerate viable microorganisms. These methods are tailored to accommodate different sample compositions and environmental conditions. The benefits of testing include improved product safety, enhanced quality assurance, and compliance with international regulatory guidelines. It aids in detecting potential contamination sources, optimizing sterilization processes, and reducing risks associated with defective or unsafe products.
Applications of bioburden testing encompass sterility assurance in medical devices, validation of aseptic processes, and evaluation of raw materials in manufacturing. This testing is also integral to pharmaceutical production, ensuring the safety and efficacy of drugs. End-use industries implementing include pharmaceuticals, biotechnology, medical devices, and food production. These industries rely on bioburden testing to safeguard public health, maintain brand reputation, and meet stringent regulatory requirements.
Key Drivers:
Rising Demand for Sterile and Contaminant-Free Medical Devices Leads to New Bioburden Testing Market Trends
The increasing focus on ensuring the sterility and safety of medical devices is a major driver for the market. Medical devices, such as implants, surgical instruments, and diagnostic equipment, must be free from harmful microbial contamination to prevent infections and complications. Bioburden testing is essential in verifying the microbial load on these devices, ensuring their safety for patients. For example, the use of bioburden testing in surgical implant manufacturing guarantees that devices like knee and hip implants meet strict cleanliness standards before being used in surgeries. As the trend for high-quality, infection-free medical devices continues to rise, the need for testing will further drive the bioburden testing market growth.
Key Restraints:
High Costs of Bioburden Testing Procedures Causes Slow Bioburden Testing Market Expansion
The high cost of bioburden testing presents a significant challenge for the market. Testing requires specialized equipment, skilled personnel, and time-consuming processes to ensure accurate results. The need for certified laboratories and compliance with regulatory standards increases operational costs, making the testing process expensive. For smaller medical device manufacturers or pharmaceutical companies with limited budgets, these costs can be prohibitive, leading to delays in product development or reduced testing frequencies. As a result, the financial burden associated with testing may limit its widespread adoption, particularly among smaller companies, thereby restraining bioburden testing market growth.
Future Opportunities :
Advancements in Rapid Bioburden Testing Technologies Create New Bioburden Testing Market Opportunities
The future of the market is poised for significant growth with advancements in rapid testing technologies. Traditional testing methods, such as culture-based techniques, can take several days to yield results. However, innovations in molecular biology and automated systems are enabling faster, more accurate testing methods. For instance, PCR-based assays and enzymatic detection systems allow for quicker detection of microbial contamination, reducing turnaround times significantly. As industries, especially medical devices and pharmaceuticals, continue to seek faster, more efficient testing solutions, the trend for these rapid testing technologies will grow, offering substantial bioburden testing market opportunities. Therefore, the development and adoption of these advanced testing methods will provide a major growth avenue for the market.
Bioburden Testing Market Segmental Analysis :
By Product Type:
Based on product type, the market is segmented into consumables, instruments, and services.
The consumables segment accounted for the largest revenue in Bioburden Testing Market share in 2024 and is anticipated to register the fastest CAGR during the forecast period.
- Consumables include media, reagents, and test kits used in bioburden testing.
- These products are critical in microbiological analysis for sterilization and quality control processes in pharmaceutical, medical devices, and food industries.
- The increasing trend for sterilization in drug production and medical device manufacturing is fueling the need for consumables.
- Additionally, advancements in reagent formulations that improve test efficiency and reduce time are contributing to their widespread adoption.
- As the pharmaceutical and biotechnology sectors continue to expand, the consumables segment will continue to drive revenue growth.
- Therefore, according to bioburden testing market analysis, the consumables segment remains the largest and fastest-growing segment, largely driven by the increasing bioburden testing market demand for sterile products in pharmaceuticals and healthcare sectors.
By Test Type:
Based on test type, the market is segmented into culture-based methods and non-culture-based methods.
The culture-based methods segment accounted for the largest revenue in Bioburden Testing Market share in 2024.
- Culture-based methods are the traditional methods of microbial testing, involving the growth of microorganisms on nutrient media to identify microbial contamination.
- These methods are widely used in pharmaceutical and biopharmaceutical companies for ensuring product sterility.
- Despite newer technologies, culture-based methods remain the gold standard due to their accuracy and reliability.
- However, they require longer timeframes, which is one of the limitations driving the growth of non-culture-based methods.
- Therefore, according to bioburden testing market analysis, culture-based methods continue to dominate the market
The non-culture-based methods segment is anticipated to register the fastest CAGR during the forecast period.
- Non-culture-based methods include techniques like PCR (Polymerase Chain Reaction), ATP testing, and flow cytometry.
- These methods offer faster results, which is a significant advantage in sectors like food & beverage and medical devices.
- As technology advances, non-culture-based methods are increasingly being favored for their rapid and accurate results.
- These methods are particularly relevant in high-throughput environments and where time efficiency is crucial.
- Thus, according to market analysis, Non-culture-based methods are experiencing rapid growth due to their speed and precision in applications across industries.
By Application:
Based on application, the market is segmented into pharmaceutical & biopharmaceutical, medical devices, food & beverage, cosmetics & personal care, and others.
The pharmaceutical & biopharmaceutical segment accounted for the largest revenue share in 2024 and is anticipated to register the fastest CAGR during the forecast period.
- The increasing number of drug production processes and regulatory pressure for sterility assurance drives the trend for bioburden testing in this sector.
- Rigorous quality control measures, including bioburden testing, are required to meet the stringent regulations set by health authorities like the FDA and EMA.
- The growing adoption of biologics and personalized medicine further elevates the need for bioburden testing to ensure the safety and quality of these products.
- As the biopharmaceutical industry expands, the trend for effective microbial control solutions will continue to rise, driving market growth.
- Therefore, according to market analysis, the pharmaceutical & biopharmaceutical application remains dominant and is expected to see continued expansion, driven by the need for stringent sterility testing and quality assurance in drug manufacturing processes.
By Method:
Baes on the method, the market is segmented into manual testing and automated testing.The manual testing segment accounted for the largest revenue share in 2024 and is anticipated to grow at a moderate pace during the forecast period.
- Manual testing involves traditional methods where technicians perform procedures manually, requiring significant time and effort.
- While manual testing is more labor-intensive, it continues to be widely used in industries where manual oversight is a necessity.
- However, its slow process and the need for skilled personnel are challenges driving innovation in automated testing.
- Therefore, according to market analysis, manual testing remains dominant.
The automated testing segment is anticipated to register the fastest CAGR during the forecast period.
- Automated testing offers faster, more reliable, and more efficient bioburden testing, reducing human error and labor costs.
- These systems are especially useful in high-volume environments, such as pharmaceutical production lines and large-scale research facilities.
- With growing emphasis on productivity and accuracy, automated systems are becoming the preferred choice for testing in regulated industries.
- Thus, according to market analysis, the shift towards automated testing is expected to accelerate as industries seek to improve efficiency and reduce labor costs.
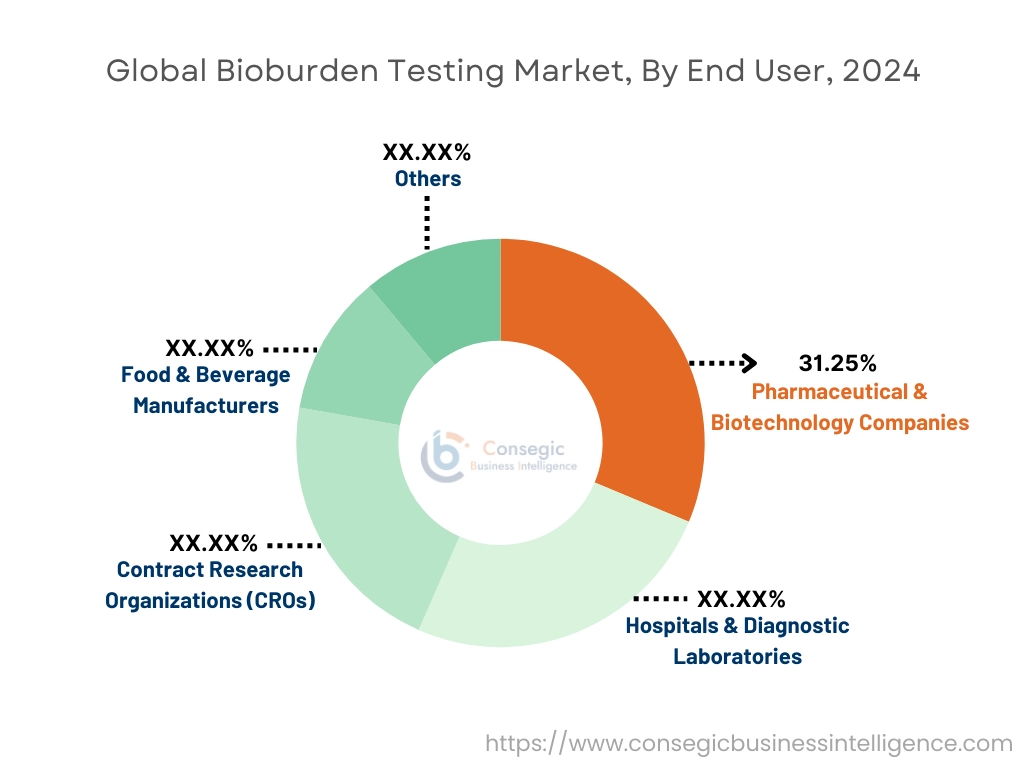
By End-User:
Based on the end-user, the market is segmented into contract research organizations (CROs), hospitals & diagnostic laboratories, pharmaceutical & biotechnology companies, food & beverage manufacturers, and others.
The pharmaceutical & biotechnology companies segment accounted for the largest revenue share by 31.25% in 2024.
- This segment encompasses companies involved in drug development, biologics production, and vaccine manufacturing, all of which require bioburden testing to ensure product sterility and safety.
- Regulatory pressures and quality assurance requirements have led pharmaceutical companies to integrate bioburden testing into their manufacturing and R&D processes.
- The increasing demand for novel therapies and biologics is fueling the growth of this segment.
- Therefore, according to market analysis, government initiatives promoting drug safety and quality are further driving the trend for testing services in this segment.
Contract Research Organizations (CROs) are anticipated to register the fastest CAGR during the forecast period.
- CROs provide outsourced research and testing services to pharmaceutical and biotechnology companies, which are increasingly outsourcing bioburden testing to reduce costs and improve efficiency.
- These organizations benefit from advancements in testing technologies, which enhance service offerings and reduce turnaround times.
- As the trend for contract services rises globally, CROs are positioned to experience substantial growth in bioburden testing services.
- Thus, according to market analysis, pharmaceutical & biotechnology companies continue to dominate the market, while the CRO segment is expanding rapidly due to increasing outsourcing trends in research and development activities.
Regional Analysis:
The regional segment includes North America, Europe, Asia Pacific, Middle East and Africa, and Latin America.
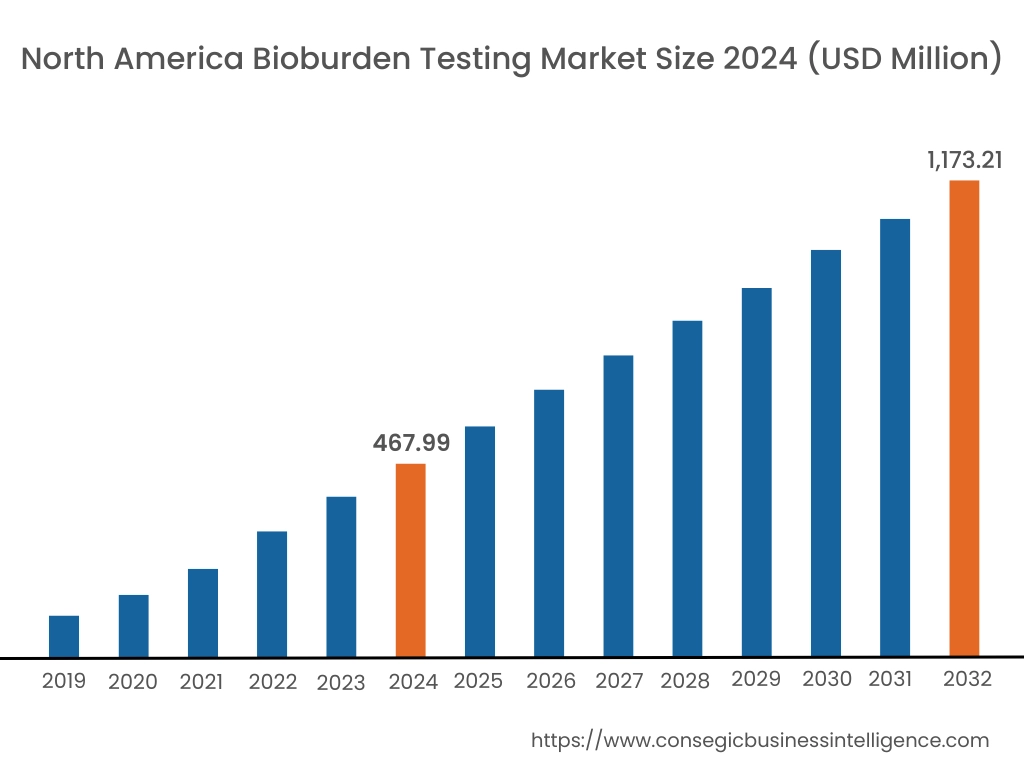
In 2024, North America was valued at USD 467.99 Million and is expected to reach USD 1,173.21 Million in 2032. In North America, the U.S. accounted for the highest share of 71.15% during the base year of 2024. North America is a key region for the bioburden testing industry, with significant demand stemming from the pharmaceutical, biotechnology, and medical device industries. Stringent regulatory standards in the United States and Canada require thorough testing to ensure product safety and compliance. The presence of leading players in the region further contributes to bioburden testing market expansion. Increasing investments in healthcare research and development also support the growing need for accurate microbial testing in the region.
In Asia Pacific, the market is experiencing the fastest growth with a CAGR of 13.0% over the forecast period. Asia-Pacific is witnessing rapid development in the bioburden testing industry, primarily driven by the growing pharmaceutical and healthcare industries in countries like China, India, and Japan. The region’s rising focus on improving healthcare infrastructure and stringent regulatory requirements for microbial testing enhance bioburden testing market demand. Additionally, increased research activities in biotechnology and life sciences create a strong market for testing services and products. However, the market is challenged by varied regulatory standards across countries in the region.
Europe holds a significant share in the bioburden testing market, supported by strong regulations on pharmaceutical and medical device safety. The European Medicines Agency (EMA) and other regulatory bodies enforce strict bioburden testing protocols for the healthcare sector. Countries such as Germany, France, and the UK prioritize compliance with these regulations, which fuels the demand for advanced testing solutions. The region’s well-established pharmaceutical industry also drives market growth, with increasing focus on product sterilization and contamination control.
The bioburden testing market in the Middle East and Africa is expanding, though at a slower pace compared to other regions. The healthcare and pharmaceutical industries are steadily growing in countries such as the UAE, Saudi Arabia, and South Africa, which increases the need for bioburden testing services. Growing awareness regarding safety standards and contamination control in the medical and pharmaceutical industries supports market development. However, limited infrastructure and regulatory challenges hinder the widespread adoption of advanced testing solutions in the region.
Latin America’s bioburden testing market is evolving, with a rising demand in the pharmaceutical, biotechnology, and medical device sectors. Brazil and Mexico are at the forefront of the market, supported by increasing investments in healthcare and research. Regulatory changes and growing awareness of contamination risks in medical and pharmaceutical products are contributing to market expansion. However, the market faces challenges related to high costs, limited access to advanced testing technologies, and regulatory inconsistencies across countries in the region.
Top Key Players & Market Share Insights:
The Global Bioburden Testing Market is highly competitive with major players providing products and services to the national and international markets. Key players are adopting several strategies in research and development (R&D), product innovation, and end-user launches to hold a strong position in the Global Bioburden Testing Market. Key players in the Bioburden Testing Market include-
- Charles River Laboratories International, Inc. (United States)
- Merck KGaA (Germany)
- Danaher Corporation (United States)
- Hach Company (United States)
- Pall Corporation (United States)
- BioMerieux S.A. (France)
- Thermo Fisher Scientific Inc. (United States)
- WuXi AppTec (China)
- Lonza Group (Switzerland)
- Eppendorf AG (Germany)
Bioburden Testing Market Report Insights:
| Report Attributes | Report Details |
| Study Timeline | 2019-2032 |
| Market Size in 2032 | USD 3,619.89 Million |
| CAGR (2025-2032) | 12.5% |
| By Product Type |
|
| By Test Type |
|
| By Application |
|
| By Method |
|
| By End-User |
|
| By Region |
|
| Key Players |
|
| North America | U.S. Canada Mexico |
| Europe | U.K. Germany France Spain Italy Russia Benelux Rest of Europe |
| APAC | China South Korea Japan India Australia ASEAN Rest of Asia-Pacific |
| Middle East and Africa | GCC Turkey South Africa Rest of MEA |
| LATAM | Brazil Argentina Chile Rest of LATAM |
| Report Coverage |
|
Key Questions Answered in the Report
How big is the Bioburden Testing Market? +
In 2024, the Bioburden Testing Market was USD 1,410.85 million.
What will be the potential market valuation for the Bioburden Testing Market by 2032? +
In 2032, the market size of Bioburden Testing Market is expected to reach USD 3,619.89 million.
What are the segments covered in the Bioburden Testing Market report? +
The product type, test type, application, method, and end-user are the segments covered in this report.
Who are the major players in the Bioburden Testing Market? +
Charles River Laboratories International, Inc. (United States), Merck KGaA (Germany), BioMerieux S.A. (France), Thermo Fisher Scientific Inc. (United States), WuXi AppTec (China), Lonza Group (Switzerland), Eppendorf AG (Germany), Danaher Corporation (United States), Hach Company (United States), Pall Corporation (United States), are the major players in the Bioburden Testing market.