- Summary
- Table Of Content
- Methodology
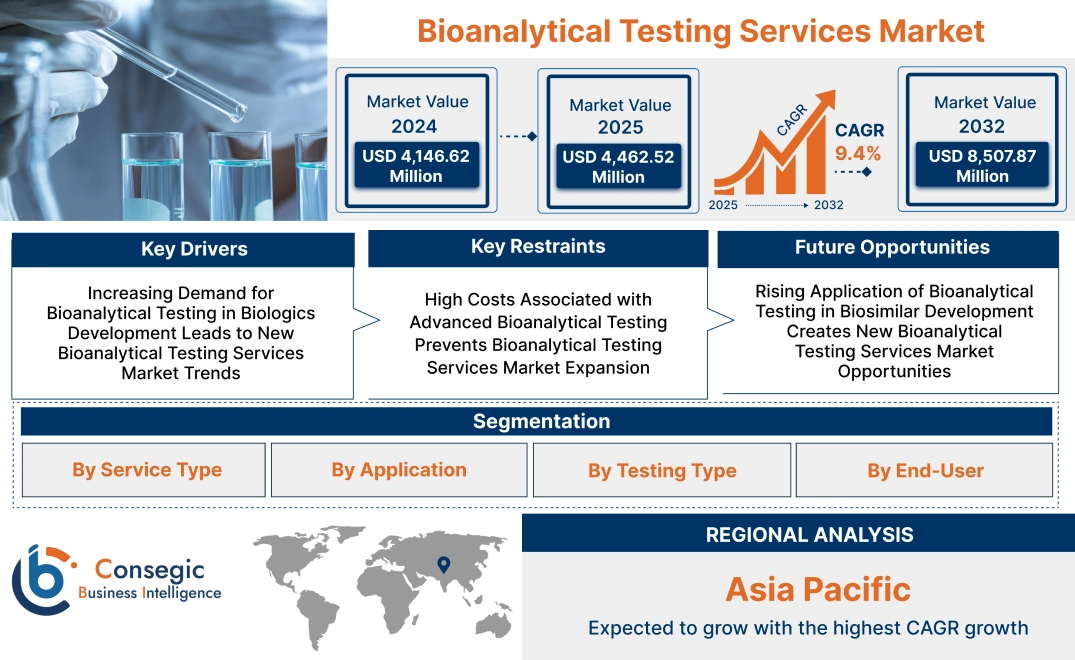
Bioanalytical Testing Services Market Size:
Bioanalytical Testing Services Market size is estimated to reach over USD 8,507.87 Million by 2032 from a value of USD 4,146.62 Million in 2024 and is projected to grow by USD 4,462.52 Million in 2025, growing at a CAGR of 9.4% from 2025 to 2032.
Bioanalytical Testing Services Market Scope & Overview:
Bioanalytical testing services encompass analytical methods and procedures used to quantify biological molecules in drug development, diagnostics, and clinical research. These services are essential for evaluating the efficacy, safety, and pharmacokinetics of pharmaceuticals, biologics, and biosimilars. Key features of bioanalytical testing include precision, sensitivity, and reliability in detecting biomolecules, metabolites, and biomarkers. Techniques such as liquid chromatography-mass spectrometry (LC-MS), enzyme-linked immunosorbent assay (ELISA), and cell-based assays are widely used to ensure accurate analysis. These services adhere to strict regulatory standards, ensuring high-quality results for compliance and validation. The benefits of bioanalytical testing services include accelerated drug development timelines, enhanced data accuracy, and cost-efficiency in conducting critical analyses. They support detailed assessment of therapeutic candidates, ensuring optimal dosage and formulation. Outsourcing these services reduces operational burdens on pharmaceutical and biotechnology companies.
Applications of bioanalytical testing extend across preclinical and clinical trials, bioavailability and bioequivalence studies, and biomarker discovery. It is pivotal in the development of biologics, vaccines, and advanced therapies, including gene and cell-based treatments. End-use industries for bioanalytical testing services include pharmaceutical companies, biotechnology firms, contract research organizations (CROs), and academic research institutions. These services are integral to modern healthcare and life sciences sectors, promoting innovation and therapeutic advancements.
Key Drivers:
Increasing Demand for Bioanalytical Testing in Biologics Development Leads to New Bioanalytical Testing Services Market Trends
The rising focus on biologics development in the pharmaceutical and biotechnology industries is significantly boosting the market. Biologics, including monoclonal antibodies, vaccines, and recombinant proteins, require precise bioanalytical testing for preclinical and clinical trials to ensure safety, efficacy, and regulatory compliance. These services enable the accurate measurement of biomarkers, drug concentrations, and pharmacokinetics in biological matrices. For instance, the growing adoption of bioanalytical testing for monoclonal antibody therapies in oncology has become a crucial step in drug approval processes. The critical role of bioanalytical testing in biologics development is driving the expansion of the market.
Key Restraints:
High Costs Associated with Advanced Bioanalytical Testing Prevents Bioanalytical Testing Services Market Expansion
Advanced bioanalytical testing methods, including mass spectrometry and high-performance liquid chromatography (HPLC), involve significant costs for equipment, skilled professionals, and laboratory setup. These expenses are often a financial burden for small and mid-sized pharmaceutical companies and research organizations. Additionally, maintaining the accuracy and reliability of bioanalytical testing requires regular calibration and validation, adding to the operational costs. For example, the implementation of liquid chromatography-mass spectrometry (LC-MS) for large-molecule analysis requires extensive investment in instrumentation and training, deterring its adoption among smaller entities. Consequently, the high cost of advanced testing technologies poses a barrier to the widespread adoption of bioanalytical testing services.
Future Opportunities :
Rising Application of Bioanalytical Testing in Biosimilar Development Creates New Bioanalytical Testing Services Market Opportunities
The increasing focus on biosimilar development presents a significant opportunity for the market. Biosimilars, designed to be comparable to existing biologic drugs, require rigorous bioanalytical testing to demonstrate their similarity in terms of efficacy, safety, and quality. Emerging markets are seeing a surge in biosimilar development, fueled by patent expirations of biologic drugs and the need for cost-effective alternatives. For example, the growing production of biosimilar insulin for diabetes management underscores the importance of bioanalytical testing in ensuring regulatory compliance. As the trend for biosimilars grows, bioanalytical testing services are expected to become increasingly critical in supporting their development and approval processes.
Bioanalytical Testing Services Market Segmental Analysis :
By Service Type:
Based on service type, the market is segmented into clinical testing services and non-clinical testing services.
The clinical testing services sector accounted for the largest revenue in Bioanalytical Testing Services Market share in 2024.
- Clinical testing services include testing related to clinical trials for drug development, diagnostics, and therapeutic monitoring.
- This service is critical for pharmaceutical and biopharmaceutical companies seeking to evaluate the safety and efficacy of new treatments.
- The rise in clinical trials and regulatory requirements are fueling the trend for these services.
- Clinical testing services also provide robust data supporting drug approvals and market entry, contributing to their significant share in the market.
- Therefore, clinical testing services remain integral to the bioanalytical testing market.
- Therefore, according to bioanalytical testing services market analysis, clinical testing services continue to dominate the market, driven by their essential role in drug development and regulatory compliance.
The non-clinical testing services sector is anticipated to register the fastest CAGR during the forecast period.
- Non-clinical testing includes preclinical trials that focus on drug safety and toxicity evaluations.
- Increasing focus on early-stage drug development and the growing use of animal models for drug testing are contributing to the bioanalytical testing services market demand for these services.
- Non-clinical testing services offer cost-effective methods for testing before clinical trials, which makes them indispensable in the development pipeline.
- With rising drug discovery efforts, the non-clinical testing segment is expected to see significant bioanalytical testing services market growth in the coming years.
- Thus, according to bioanalytical testing services market analysis, the non-clinical testing sector is expected to experience rapid growth due to the increasing importance of early-stage drug safety testing and the t for preclinical evaluations.
By Application:
Based on application, the market is segmented into pharmaceuticals, biopharmaceuticals, medical devices, cosmetics, and others.
The pharmaceuticals sector accounted for the largest revenue in Bioanalytical Testing Services Market share in 2024.
- Pharmaceuticals require bioanalytical testing for drug development, clinical trials, and regulatory submissions.
- Increasing pharmaceutical R&D spending and the need for precise data to ensure drug efficacy and safety are driving this segment.
- The growing number of new drug approvals and the increasing complexity of formulations further contribute to the pharmaceutical sector’s dominance.
- As a result, the pharmaceuticals sector continues to lead the bioanalytical testing services market.
- Thus, according to market analysis, the pharmaceutical sector remains the dominant force in the bioanalytical testing services market, driven by extensive R&D activities and regulatory needs.
The biopharmaceuticals sector is anticipated to register the fastest CAGR during the forecast period.
- The increasing number of biologic drugs, including monoclonal antibodies and gene therapies, requires specialized testing.
- Bioanalytical testing services are essential for biopharmaceutical companies to assess complex biologics, ensuring product quality and safety.
- As the biopharmaceutical industry continues to expand, the trend for these services in this sector is expected to rise rapidly.
- Thus, according to market analysis, the biopharmaceutical sector is poised for the fastest growth, fueled by the rise of biologics and the need for specialized bioanalytical testing services.
By Testing Type:
Based on testing type, the market is segmented into ADME-Tox testing, pharmacokinetics (PK) and toxicokinetics (TK) testing, biomarker analysis, bioequivalence testing, biosafety testing, and others.
The ADME-Tox testing sector accounted for the largest revenue share in 2024.
- ADME-Tox testing involves evaluating the absorption, distribution, metabolism, excretion, and toxicity of potential drug candidates.
- The rising emphasis on early-stage drug testing to minimize adverse effects and the need for more efficient drug development are key factors contributing to this segment’s dominance.
- Additionally, ADME-Tox testing provides critical data to understand how drugs interact with the body, improving safety outcomes for pharmaceutical products.
- Thus, ADME-Tox testing remains a core component of the bioanalytical testing services market.
- Therefore, according to market analysis, ADME-Tox testing remains central to bioanalytical testing, offering crucial data that supports drug safety and efficacy during early drug development.
The biosafety testing sector is anticipated to register the fastest CAGR during the forecast period.
- Biosafety testing ensures the safety of pharmaceutical, biologic, and medical device products, crucial in preventing harmful effects.
- As biologics and vaccines become increasingly prevalent, the trend for comprehensive safety testing has surged.
- Biosafety testing also helps meet regulatory requirements and establishes the safety profile of new products, driving its rapid growth.
- With expanding healthcare needs, this sector is poised for significant growth in the coming years.
- Thus, according to market analysis, the biosafety testing sector is expected to grow rapidly, driven by the increased trend for safety assessments of biologics and vaccines.
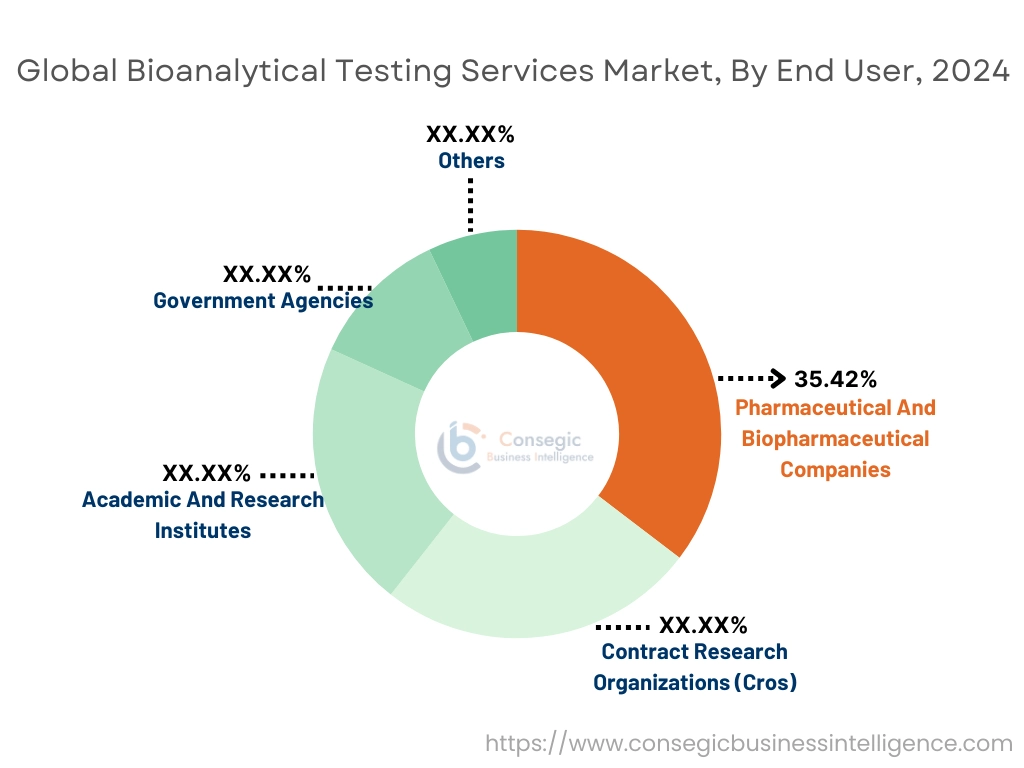
By End-User:
Based on end-user, the market is segmented into contract research organizations (CROs), pharmaceutical and biopharmaceutical companies, academic and research institutes, government agencies, and others.
The pharmaceutical and biopharmaceutical companies sector accounted for the largest revenue share by 35.42% in 2024.
- These companies require bioanalytical testing services for drug development, clinical trials, and post-market surveillance.
- The expansion of drug pipelines and the increasing regulatory burden are driving the demand for testing services in this sector.
- Pharmaceutical and biopharmaceutical companies rely on accurate and timely bioanalytical data for successful product development, contributing to the sector’s leading position.
- Therefore, pharmaceutical and biopharmaceutical companies are the largest users of bioanalytical testing services.
- Therefore, according to market analysis, pharmaceutical and biopharmaceutical companies continue to be the largest users of bioanalytical testing services, as they rely on these services to ensure the safety and efficacy of their products.
The contract research organizations (CROs) sector is anticipated to register the fastest CAGR during the forecast period.
- CROs offer testing services on behalf of pharmaceutical, biopharmaceutical, and medical device companies, helping them reduce costs and time.
- The increasing outsourcing of clinical trials and laboratory testing to CROs is expected to drive the bioanalytical testing services market demand in this segment.
- As the reliance on CROs continues to rise, this segment is forecast to experience robust growth over the forecast period.
- Thus, according to market analysis, the CRO sector is set for rapid growth, driven by the outsourcing trend and the increasing demand for cost-effective testing solutions.
Regional Analysis:
The regional segment includes North America, Europe, Asia Pacific, Middle East and Africa, and Latin America.
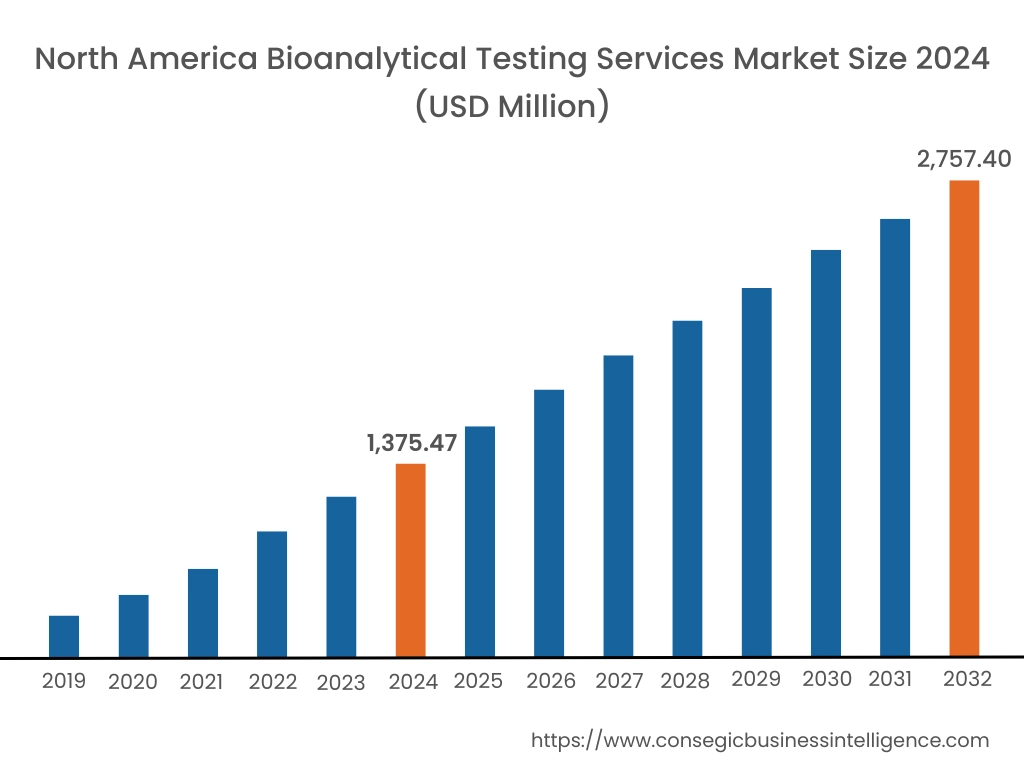
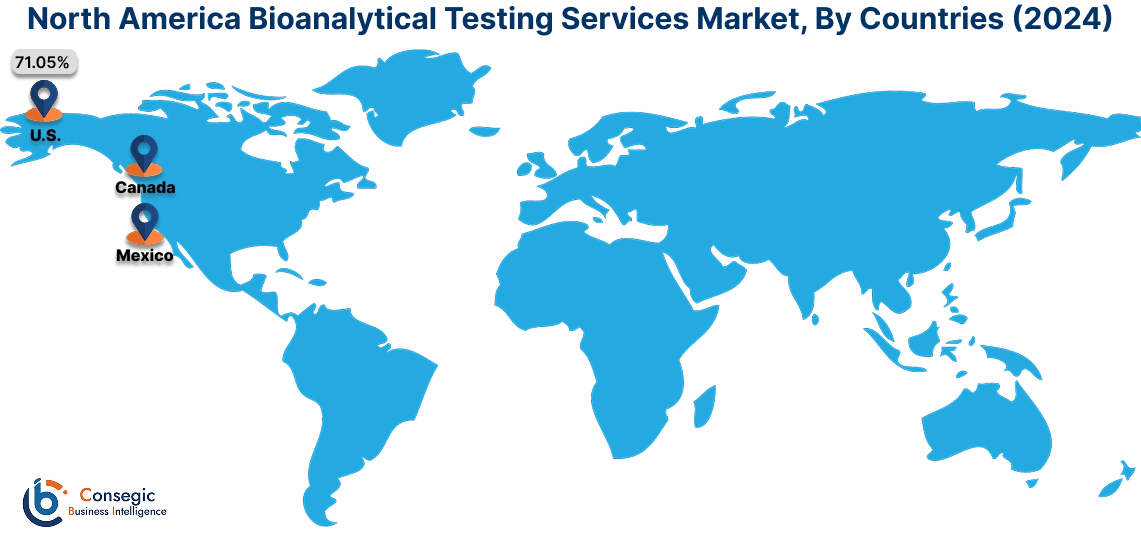
In 2024, North America was valued at USD 1,375.47 Million and is expected to reach USD 2,757.40 Million in 2032. In North America, the U.S. accounted for the highest share of 71.05% during the base year of 2024. North America holds a significant share of the bioanalytical testing services industry. The presence of leading service providers, along with advanced healthcare infrastructure, strengthens the market. High demand for clinical trials, particularly in the pharmaceutical sector, drives the need for bioanalytical testing services. Moreover, strict regulatory standards and a focus on personalized medicine are key factors influencing market performance.
In Asia Pacific, the market is experiencing the fastest growth with a CAGR of 9.9% over the forecast period.
The Asia-Pacific region experiences substantial demand for bioanalytical testing services, primarily due to increasing healthcare investments and expanding pharmaceutical industries in countries like China and India. The growing outsourcing trend, along with cost-effective testing services, encourages market expansion. Government initiatives to improve healthcare infrastructure and enhance clinical trial capabilities support the region's market development.
Europe benefits from robust healthcare systems and advanced research in bioanalytical testing. The region's pharmaceutical and biotechnology industries heavily rely on these services for drug development and clinical trials. Increased focus on biologics and personalized medicine further accelerates the demand for bioanalytical testing services. Stringent regulatory requirements and high healthcare standards contribute to the market's growth in this region.
In the Middle East and Africa, the bioanalytical testing services industry is evolving, supported by improving healthcare infrastructure and rising pharmaceutical activities. Investments in clinical trials and a growing interest in outsourcing research and testing services are key factors influencing market development. The presence of healthcare hubs in countries like the UAE and South Africa aids the market's progress in this region.
The Latin American market for bioanalytical testing services is witnessing steady growth, with countries like Brazil and Mexico seeing increased demand for clinical trials and pharmaceutical testing. The expansion of the healthcare sector and growing pharmaceutical research in the region are key factors shaping the market. Cost-effectiveness and increasing partnerships with global service providers enhance the market outlook.
Top Key Players & Market Share Insights:
The Global Bioanalytical Testing Services Market is highly competitive with major players providing products and services to the national and international markets. Key players are adopting several strategies in research and development (R&D), product innovation, and end-user launches to hold a strong position in the Global Bioanalytical Testing Services Market. Key players in the Bioanalytical Testing Services industry include-
- Labcorp Drug Development (United States)
- Quotient Sciences (United Kingdom)
- PPD, Inc. (United States)
- Covance Inc. (United States)
- Celerion (United States)
- Charles River Laboratories (United States)
- WuXi AppTec (China)
- Eurofins Scientific (Luxembourg)
- Syneos Health (United States)
- Medpace (United States)
Bioanalytical Testing Services Market Report Insights:
| Report Attributes | Report Details |
| Study Timeline | 2019-2032 |
| Market Size in 2032 | USD 8,507.87 Million |
| CAGR (2025-2032) | 9.4% |
| By Service Type |
|
| By Application |
|
| By Testing Type |
|
| By End-User |
|
| By Region |
|
| Key Players |
|
| North America | U.S. Canada Mexico |
| Europe | U.K. Germany France Spain Italy Russia Benelux Rest of Europe |
| APAC | China South Korea Japan India Australia ASEAN Rest of Asia-Pacific |
| Middle East and Africa | GCC Turkey South Africa Rest of MEA |
| LATAM | Brazil Argentina Chile Rest of LATAM |
| Report Coverage |
|
Key Questions Answered in the Report
How big is the Bioanalytical Testing Services Market? +
In 2024, the Bioanalytical Testing Services Market was USD 4,146.62 million.
What will be the potential market valuation for the Bioanalytical Testing Services Market by 2032? +
In 2032, the market size of Bioanalytical Testing Services Market is expected to reach USD 8,507.87 million.
What are the segments covered in the Bioanalytical Testing Services Market report? +
The service type, application, testing type, and end-user are the segments covered in this report.
Who are the major players in the Bioanalytical Testing Services Market? +
Labcorp Drug Development (United States), Quotient Sciences (United Kingdom), Charles River Laboratories (United States), WuXi AppTec (China), Eurofins Scientific (Luxembourg), Syneos Health (United States), Medpace (United States), PPD, Inc. (United States), Covance Inc. (United States), Celerion (United States) are the major players in the Bioanalytical Testing Services market.