- Summary
- Table Of Content
- Methodology
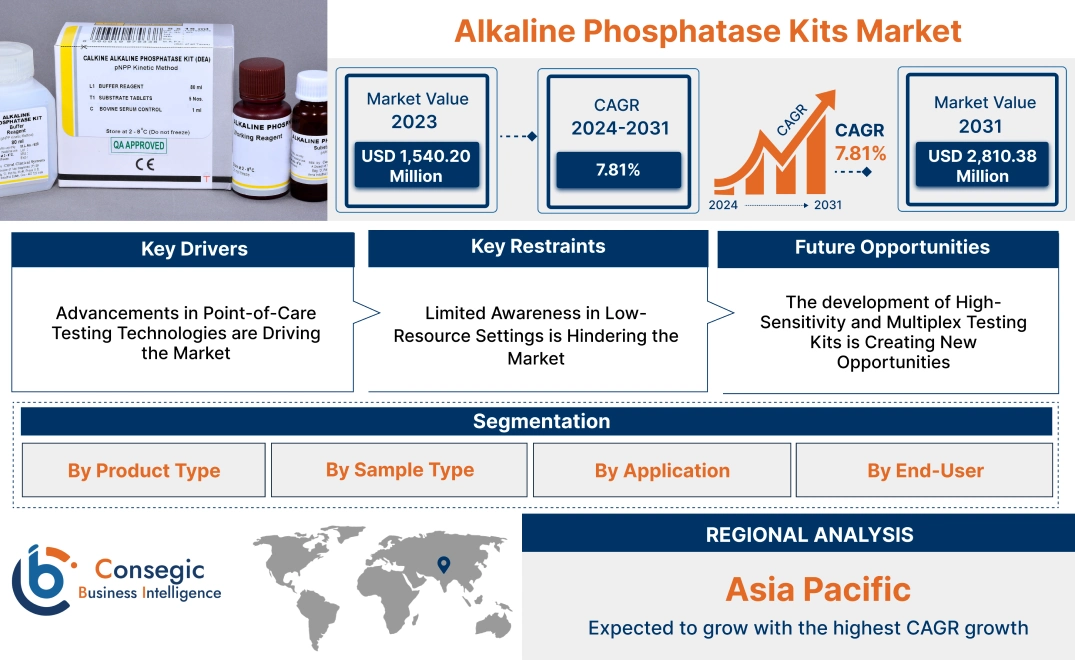
Alkaline Phosphatase Kits Market Size:
Alkaline Phosphatase Kits Market size is estimated to reach over USD 2,810.38 Million by 2031 from a value of USD 1,540.20 Million in 2023 and is projected to grow by USD 1,633.01 Million in 2024, growing at a CAGR of 7.81% from 2024 to 2031.
Alkaline Phosphatase Kits Market Scope & Overview:
The alkaline phosphatase kits are used to detect and measure alkaline phosphatase (ALP) enzyme activity in biological samples. These kits are widely utilized in clinical diagnostics, biotechnology, and pharmaceutical research to monitor liver function, bone disorders, and metabolic activities. Key characteristics of alkaline phosphatase kits include high sensitivity, specificity, and compatibility with various sample types such as serum, plasma, and tissue extracts. The benefits include rapid and accurate results, ease of use, and reliability for both qualitative and quantitative analyses. Applications span clinical diagnostics for liver and bone health, enzyme-linked immunosorbent assays (ELISA), and drug development research. End-users include hospitals, diagnostic laboratories, academic research institutions, and biotechnology companies, driven by the increasing prevalence of liver and bone diseases, advancements in enzyme detection technologies, and growing demand for precision diagnostics.
Key Drivers:
Advancements in Point-of-Care Testing Technologies are Driving the Market
The integration of point-of-care (POC) testing technologies into alkaline phosphatase (ALP) diagnostic workflows has revolutionized accessibility and efficiency in healthcare. POC-enabled ALP kits are designed to provide rapid and accurate results directly at the site of patient care, eliminating the need for centralized laboratory facilities. These portable and user-friendly kits are particularly valuable in outpatient clinics, remote locations, and emergency settings, where timely diagnosis is critical.
Trends in decentralized healthcare and technological innovation are driving the adoption of POC solutions across diverse medical environments. By incorporating advanced features such as digital interfaces, automated calibration, and cloud-based data sharing, POC ALP kits are enhancing diagnostic accuracy and efficiency. As the analysis highlights, these advancements are transforming traditional diagnostic approaches and enabling broader access to critical testing.
Key Restraints :
Limited Awareness in Low-Resource Settings is Hindering the Market
Despite the essential role of alkaline phosphatase testing in diagnosing various health conditions, limited awareness and diagnostic infrastructure in low-resource settings present significant challenges. Many healthcare providers in these regions lack access to reliable testing kits, trained personnel, and standardized procedures, leading to underutilization of ALP diagnostics.
This lack of awareness extends to patients, who may not recognize the importance of routine enzyme testing for early disease detection. Addressing these gaps requires concerted efforts in education, training, and capacity building. Trends in global health initiatives and partnerships with local organizations offer alkaline phosphatase kits market opportunities to enhance awareness and accessibility, ensuring that ALP diagnostics reach underserved populations.
Future Opportunities :
The development of High-Sensitivity and Multiplex Testing Kits is Creating New Opportunities
The ongoing advancements in diagnostic technologies have paved the way for the development of high-sensitivity alkaline phosphatase kits. These kits are capable of detecting even minute changes in enzyme levels, improving their utility in early diagnosis and monitoring of complex conditions such as liver, bone, and kidney disorders. High-sensitivity kits are particularly valuable in personalized medicine, where precise biomarker analysis is critical.
Additionally, multiplex testing kits that combine ALP testing with other biomarkers in a single assay are streamlining diagnostic workflows. These integrated solutions reduce the time and resources required for comprehensive testing, aligning with trends in efficient and patient-centric healthcare. By focusing on innovation and adaptability, manufacturers have an opportunity to address the evolving needs of healthcare providers while enhancing the clinical relevance of ALP diagnostics.
Alkaline Phosphatase Kits Market Segmental Analysis :
By Product Type:
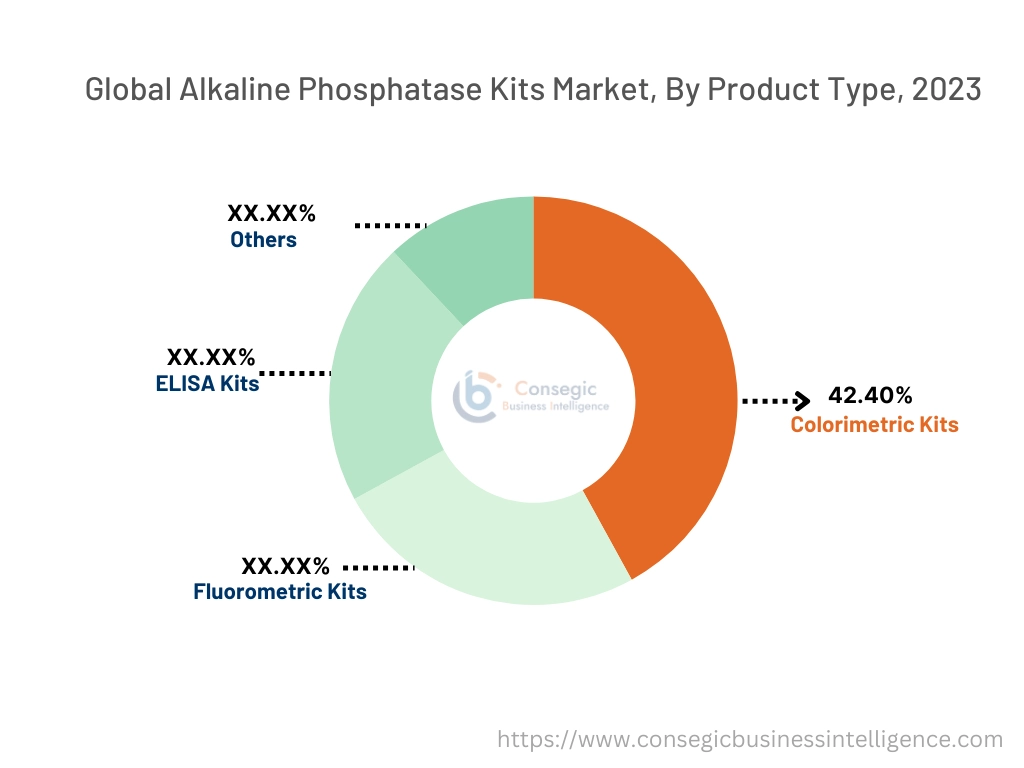
Based on Product type the market is segmented into Colorimetric Kits, Fluorometric Kits, ELISA Kits, and Others.
Colorimetric Kits held the largest revenue of 42.40% share in the alkaline phosphatase kits market in 2023.
- The dominance of colorimetric kits is attributed to their cost-effectiveness, ease of use, and widespread availability for routine laboratory applications.
- These kits are extensively used in biochemical assays, clinical diagnostics, and research laboratories, making them a staple in detecting enzyme activity and quantifying analytes.
- Their compatibility with standard laboratory equipment, such as spectrophotometers, further enhances their adoption across diverse applications.
- Advancements in reagent stability and assay sensitivity have bolstered the reliability and efficiency of colorimetric kits, ensuring their continued preference in both clinical and academic settings.
Fluorometric Kits are projected to register the fastest CAGR during the forecast period.
- Their advanced fluorescence detection capabilities enable real-time, highly sensitive measurements, particularly for low-concentration analytes.
- Fluorometric kits are increasingly favored for applications in cancer research, enzyme activity studies, and drug discovery.
- Technological advancements, including time-resolved fluorescence and FRET, have expanded their scope of use, driving growth in academic and industrial research settings.
- The rising adoption of precision diagnostic tools is further accelerating the growth of fluorometric kits, positioning them as a rapidly emerging segment in the market.
By Sample Type:
Based on sample type, the market is segmented into Urine, Plasma, Serum, and Cell Culture Supernatant.
Plasma samples accounted for the largest revenue in alkaline phosphatase kits market share in 2023.
- Plasma is highly valued in diagnostic and therapeutic research due to its ability to provide comprehensive insights into metabolic and pathological conditions.
- It is a key sample type in biomarker discovery, therapeutic drug monitoring, and disease progression analysis.
- Additionally, plasma's stability and compatibility with advanced diagnostic platforms make it an essential component in clinical laboratories and research studies.
- Its role in liquid biopsy and personalized medicine further enhances its market significance.
- Plasma leads the sample type segment owing to its stability, diagnostic utility, and role in advancing biomarker-based research.
Cell Culture Supernatant is anticipated to exhibit the fastest growth.
- This sample type is essential for enzyme activity analysis, biologics development, and cell-based assays.
- Increasing adoption of high-throughput cell culture systems in drug discovery and protein production drives demand for cell culture supernatants.
- Moreover, their role in monoclonal antibody research and cell therapy development further accelerates alkaline phosphatase kits market growth in this segment.
- Cell Culture Supernatant is set to grow rapidly, supported by its critical applications in biologics research and drug discovery.
By Application:
Based on application, the market is segmented into Disease Diagnostics, Drug Development, Cancer Research, Enzyme Activity Measurement, and Others.
Disease Diagnostics held the largest market share in 2023
- It is driven by the rising prevalence of chronic and infectious diseases worldwide.
- Diagnostic kits play a critical role in early disease detection, improving treatment outcomes, and reducing healthcare costs.
- The integration of biomarker-based assays with AI and machine learning technologies further enhances their diagnostic accuracy and efficiency.
- The trends for point-of-care diagnostic kits and at-home testing solutions also contribute significantly to the segment's growth.
- Disease Diagnostics dominate the market due to advancements in early detection technologies and the growing need for precise diagnostic tools.
Cancer Research is anticipated to grow at the highest CAGR during the forecast period.
- The rising incidence of cancer and the increasing focus on personalized treatment approaches are driving alkaline phosphatase kits market demand for diagnostic kits in oncology research.
- Technologies such as liquid biopsy and multiplex assays are transforming cancer diagnostics, enabling early detection and monitoring of treatment efficacy.
- Additionally, growing investments in oncology research further support the adoption of advanced diagnostic solutions.
- Cancer Research is expected to grow rapidly, supported by advancements in oncology-focused diagnostic technologies and personalized medicine initiatives.
By End-User:
Based on end-user, the market is segmented into Hospitals, Research & Academic Institutes, Pharmaceutical & Biotechnology Companies, Diagnostic Laboratories, and Others.
Pharmaceutical & Biotechnology Companies held the largest revenue share in 2023.
- These companies heavily utilize diagnostic kits in drug discovery, preclinical trials, and quality control processes.
- The increasing focus on biologics and precision medicine has heightened the demand for diagnostic tools that support therapeutic development.
- Investments in R&D, particularly in the field of biomarker analysis and personalized therapies, further drive this segment's growth.
- Pharmaceutical & Biotechnology Companies lead the trends in the market due to their extensive use of diagnostic tools in R&D and clinical applications.
Research & Academic Institutes are anticipated to grow at the fastest rate during the forecast period.
- These institutes are integral to advancing diagnostic technologies and exploring novel applications for assay kits.
- Collaborations with industry players and increasing integration of diagnostic kits in academic research and training programs are significant alkaline phosphatase kits market growth drivers.
- The growing focus on innovation and education in diagnostics further supports this segment's expansion.
- Research & Academic Institutes are set to grow rapidly, driven by trends and their role in innovation, academic research, and industry collaboration.
Regional Analysis:
The regions covered are North America, Europe, Asia Pacific, the Middle East and Africa, and Latin America.
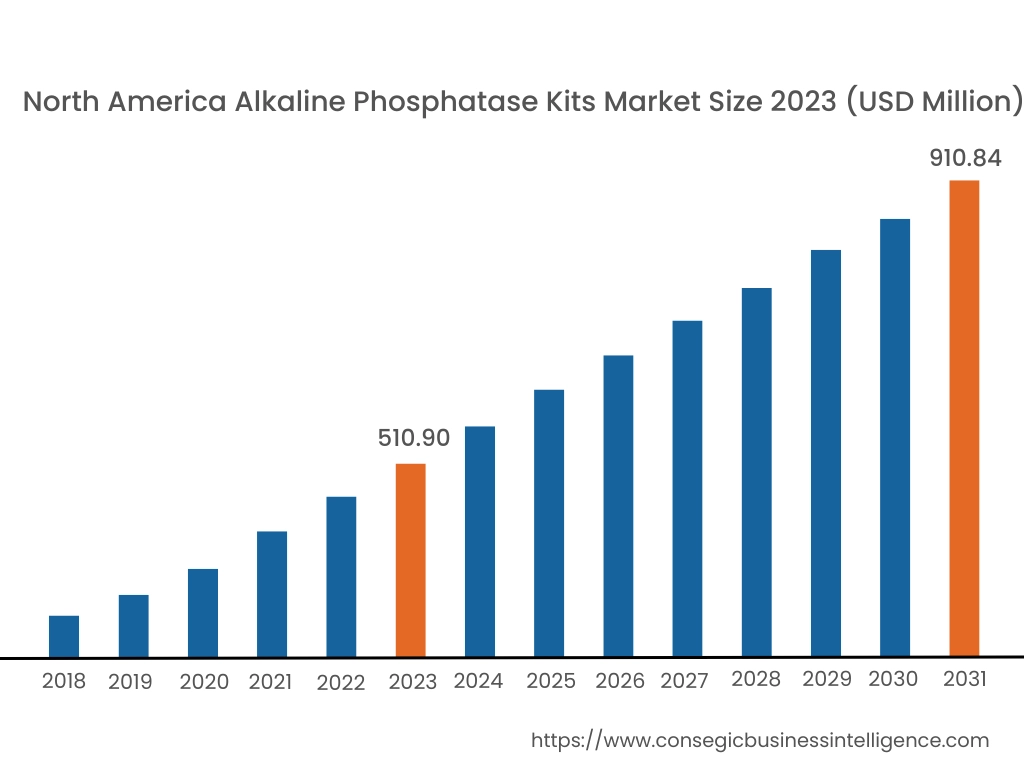
In 2023, North America was valued at USD 510.90 Million and is expected to reach USD 910.84 Million in 2031. In North America, the U.S. accounted for the highest share of 72.40% during the base year of 2023. North America holds a significant share in the alkaline phosphatase kits market analysis, driven by the well-established healthcare infrastructure and high investment in research and diagnostics. The U.S. dominates the region due to the widespread application of alkaline phosphatase kits in clinical diagnostics, drug discovery, and biotechnology research. The growing prevalence of bone, liver, and intestinal disorders further drives the alkaline phosphatase kits market demand. Canada also contributes to the market with its increasing focus on research in molecular biology and immunology. However, high costs associated with advanced diagnostic kits may limit access in some segments of the population.
In Asia Pacific, the market is experiencing the fastest growth with a CAGR of 8.3% over the forecast period. The market is fueled by the rapid expansion of healthcare and research infrastructure in China, India, and Japan. China leads the region with increasing adoption of diagnostic kits in hospitals and research institutions, driven by the growing prevalence of chronic diseases and advancements in healthcare technologies. India’s expanding pharmaceutical and biotechnology industries support the surge for alkaline phosphatase kits in drug discovery and clinical research. Japan emphasizes high-precision diagnostic tools, leveraging these kits in enzyme-linked immunoassays and molecular biology research. However, limited awareness and affordability issues in rural areas may hinder adoption in some parts of the region.
Europe is a prominent market for alkaline phosphatase kits, supported by the region's strong focus on healthcare research and advanced diagnostic technologies. Countries like Germany, France, and the UK are key contributors. Germany’s robust biopharmaceutical industry drives the use of these kits in drug development and enzyme activity studies. France emphasizes their application in clinical diagnostics, particularly for liver and bone disorders, while the UK focuses on research applications in immunoassays and enzyme-linked assays. However, stringent EU regulations on diagnostic products and high compliance costs may pose challenges for manufacturers.
The Middle East & Africa region is witnessing steady development in the alkaline phosphatase kits market, driven by increasing investments in healthcare infrastructure and rising prevalence of liver and bone disorders. The UAE and Saudi Arabia are leading market trends, adopting these kits for clinical diagnostics and research in modern healthcare facilities. In Africa, South Africa is emerging as a key market, focusing on improving diagnostics for chronic diseases and boosting research in life sciences. However, limited local manufacturing capabilities and reliance on imports may restrict broader market development in the region.
Latin America is an emerging market for alkaline phosphatase kits, with Brazil and Mexico leading the region. Brazil’s growing healthcare sector and rising prevalence of bone-related disorders drive the trends for these kits in clinical diagnostics. Mexico’s expanding biotechnology and pharmaceutical research sectors also support their adoption of enzyme-linked assays and molecular biology research. However, inconsistent regulatory frameworks and limited access to advanced diagnostic tools in some countries may hinder alkaline phosphatase kits market expansion in the region.
Top Key Players and Market Share Insights:
The Alkaline Phosphatase Kits market is highly competitive with major players providing products and services to the national and international markets. Key players are adopting several strategies in research and development (R&D), product innovation, and end-user launches to hold a strong position in the global Alkaline Phosphatase Kits market. Key players in the Alkaline Phosphatase Kits industry include -
- MilliporeSigma (U.S.)
- Abcam plc (U.K.)
- Abnova Corporation (Taiwan)
- BioAssay Systems (U.S.)
- Randox Laboratories Ltd. (U.K.)
- Siemens Healthineers (Germany)
- Thermo Fisher Scientific Inc. (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- Bio-Techne Corporation (U.S.)
- GeneTex, Inc. (U.S.)
Alkaline Phosphatase Kits Market Report Insights :
| Report Attributes | Report Details |
| Study Timeline | 2018-2031 |
| Market Size in 2031 | USD 2,810.38 Million |
| CAGR (2024-2031) | 7.81% |
| By Product Type |
|
| By Sample Type |
|
| By Application |
|
| By End-User |
|
| By Region |
|
| Key Players |
|
| North America | U.S. Canada Mexico |
| Europe | U.K. Germany France Spain Italy Russia Benelux Rest of Europe |
| APAC | China South Korea Japan India Australia ASEAN Rest of Asia-Pacific |
| Middle East and Africa | GCC Turkey South Africa Rest of MEA |
| LATAM | Brazil Argentina Chile Rest of LATAM |
| Report Coverage |
|
Key Questions Answered in the Report
What is the projected market size of the Alkaline Phosphatase Kits Market by 2031? +
Alkaline Phosphatase Kits Market size is estimated to reach over USD 2,810.38 Million by 2031 from a value of USD 1,540.20 Million in 2023 and is projected to grow by USD 1,633.01 Million in 2024, growing at a CAGR of 7.81% from 2024 to 2031.
Which product type dominates the market? +
ELISA Kits hold the largest market share due to their high sensitivity, specificity, and wide application in disease diagnostics, drug development, and biomarker analysis.
What drives the demand for alkaline phosphatase kits in disease diagnostics? +
The rising prevalence of liver and bone disorders, coupled with advancements in enzyme detection technologies, drives the demand for these kits in early and accurate disease diagnosis.
What are the key challenges faced by the market? +
Limited awareness and inadequate diagnostic infrastructure in low-resource settings are major challenges, hindering the adoption of alkaline phosphatase kits in underserved regions.